Abstract
Blue nevi are characterized by a collection of pigment-producing melanocytes in the dermis. These lesions clinically present as well demarcated cerulean-blue or bluish black colored papules or plaques that usually measure less than 1 cm in diameter. They are typically found on the dorsal surface of the hands and feet or in the head and neck region; however, they are rarely found in the oral cavity. These lesions are usually benign and stable over time. However, malignant melanomas developing in or associated with a blue nevus (which is also called malignant blue nevus) have been only rarely reported. A malignant blue nevus might develop in a common blue or cellular blue nevus, a giant congenital nevus or in a nevus of Ota, or it may be malignant from the start. Malignant blue nevi most commonly are found on the scalp. A malignant blue nevus of the lip has not been previously reported in the medical literature. We report here on a patient with a malignant melanoma associated with a blue nevus of the lip. The malignant melanoma was presumed to have developed from a blue nevus that was present on the upper lip of a 50-year-old male.
A blue nevus is a neoplasm that's composed of pigmented dendritic dermal melanocytic cells in the reticular dermis. A blue nevus can develop anywhere on the body; however, about half of the common blue nevi have been lesions on the dorsal surface of the hands and feet, as well as on the scalp, and the cellular blue nevi have been located over the buttocks. Rarely have these lesions been identified in the oral cavity, yet when they do present in the oral cavity, they have been reported in the hard palate, buccal mucosa, upper lip, lower lip and soft palate, in the order of most common frequency1. A single lesion is usually identified, but on rare occasions there may be multiple papules or nodules with satellite lesions that are mistaken for a malignant melanoma. The blue nevus lesion is considered to be completely benign, but it might show malignant transformation in rare cases2. Skin biopsy must be performed for lesions with suspicious malignant changes such as loss of a regular border and/or the development of satellite lesions. Malignant melanoma developing in or associated with a blue nevus is usually referred to as a malignant blue nevus. However, a malignant blue nevus may on rare occasions develop in a giant congenital nevus, a nevus of Ota or it may develop de novo.
We report here on a 50-year-old male patient with bluish black macules on the upper lip that had been present for 3 years; recent changes had occurred with ulcerative nodules and irregular borders, as well as satellite lesions.
A 50-year-old man presented to the dermatology clinic with a 3 year history of bluish black macules that were 3 cm in diameter on the upper lip. The skin lesions had infiltrative changes with ulcerative nodules and irregular borders with satellite lesions that had developed within the past months (Fig. 1). On the initial clinical examination, the skin lesions were localized to the upper lip and they spared the palate, buccal mucosa and lower lip. No palpable lymph nodes were detected in the head and neck region.
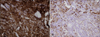
Skin biopsies were separately performed on the bluish black macule and ulcerative nodule. The histopathology of the bluish black macule (Fig. 2) showed blue nevi composed of pigmented spindle-shaped and dendritic melanocytes, and this was associated with alteration of the collagen architecture in the dermis. The heavily pigmented melanocytes were grouped in an irregular pattern among thickened collagen bundles in the reticular dermis, close to the epidermis. The histopathology of the ulcerative nodule (Fig. 3A, B) showed a malignant blue nevus composed of atypical epithelioid tumor cells with hyperchromatic and polymorphous nuclei in the dermis, spindle-shaped tumor cells containing dark melanin granules. There were more cellular epithelioid tumor cells at the bottom layer that showed frequent mitosis and focal necrosis (Fig. 3C). A diffuse proliferation of the tumor cells extended into the deep dermis. In addition, there were focal atypical large melanocytes arranged as single cells in the basal layer of the epidermis (Fig. 3D). Any pagetoid spread of the atypical melanocytes into the upper layer of the epidermis was not observed. The tumor cells were predominantly located in the dermis; the drop off sign was not seen for the tumor cells in the epidermis. Immunohistochemical stains for HMB45 and S-100 proteins (Fig. 3E, F) were positive in the tumor cells. We performed staining for CD117 (c-kit) (Fig. 4), which showed diffuse cytoplasmic staining in the bluish black macule (the blue nevus component). However, the c-kit expression was decreased in the cellular epithelioid tumor cells of the ulcerative nodule (the malignant blue nevus component).
The possibility of metastasis was ruled out by a work-up that included chest X-ray, whole body PET-CT, neck CT and gastroscopy; all the results were negative for metastasis. The laboratory chemistry tests, including lactate dehydrogenase and the liver enzyme activity, were within the normal range. Wide excision with cervical regional lymph node dissection was performed. The lymph node biopsies were negative for metastasis. However, two months later, the patient presented with a palpable mass at the left cervical region, and the mass was positive for metastasis to the lymph nodes on the subsequent biopsy (Fig. 5A). The CD117 (c-kit) staining of the metastatic lymph nodes was of strong intensity (Fig. 5B). The patient was treated with a modified radical neck lymph node dissection and parotidectomy on the left side and he has been followed for 1 year without any sign of recurrence.
Most blue nevi develop on the skin; a blue nevus in the oral cavity is rare. However, among the nevi of the oral cavity, blue nevi is the second most common form that accounts for 8.3% to 36% of all oral melanocytic nevi3,4. A blue nevus in the oral cavity typically presents between the third and fifth decades of life and the hard palate is the most frequently involved site, followed by the buccal mucosa, upper lip, lower lip and soft palate1.
Malignant melanomas of the mucosa have been reported to account for about 3~4% of all melanomas in the USA5,6. The head and neck regions are the most frequent sites of malignant melanoma of the mucosa. The nasal cavity (31.9%) is the most common site, followed by the oral cavity (23.9%), esophagus (6.3%) and lips (2.8%)6. The upper part of the oral cavity is the preferred site for a malignant melanoma of the mucosa. To date, there has been no direct association between the subtype of oral melanocytic nevi and oral malignant melanomas.
Malignant blue nevi or malignant melanomas developing in or associated with a blue nevus are extremely rare. There has been no reported data regarding the incidence of these findings, and there are no previous reports of malignant blue nevi of the oral mucosa or lip. A malignant blue nevus or malignant melanoma developing in or associated with a blue nevus had been most commonly found on the scalp7,8. Hagiwara et al.9 reported on a rare case of a 42-year-old woman with a vulvar malignant melanoma and a blue nevus of the cervix.
The common histological features of blue nevi include the presence of pigmented spindle-shaped and dendritic melanocytes in the dermis, and these melanocytes are associated with alterations of the dermal collagen architecture. There are three types of benign blue nevi: the common blue nevus, the cellular blue nevus and the combined blue nevus, in addition to malignant blue nevi. The common blue nevus is the most common subtype seen in the oral cavity1,3 and it is completely benign. However, cellular blue nevi may be locally aggressive and show persistence and recurrence after complete excision2,10,11. Furthermore, there are a few cases where cellular blue nevi have metastasized to regional lymph nodes10,11. Some studies have suggested that these cells do not represent a true metastasis, but rather, they were passively transported to the lymph nodes11. However, some of these lesions may best be interpreted as atypical cellular blue nevi or cellular blue nevi with atypical features and an indeterminate biological potential10,11. Atypical cellular blue nevi are characterized by features that include architectural atypia (an infiltrative margin and/or asymmetry) and/or cytologic atypia (hypercellularity, nuclear pleomorphism, hyperchromasia, occasional mitotic figures and/or subtle necrosis)12. The absence or scarcity of mitotic figures, necrosis and high-grade atypia is not consistent with a malignant blue nevus. The presence of areas of dendritic blue nevi type cells elsewhere in the tumor and the absence of an intraepidermal component are not suggestive of a melanoma. Malignant blue nevi show a combination of high grade atypia, spontaneous tumor necrosis and more than a few mitoses. However, some lesions do not meet all of these criteria and they show overlap with atypical cellular blue nevi with regional metastasis10,11.
There is some dispute as to whether the malignant blue nevus should be considered a separate entity from a melanoma or if it should be simply referred to as a malignant melanoma developing in or associated with a blue nevus10,11. Malignant blue nevi differ from primary melanomas by the absence of junctional activity and the presence of associated blue nevi13. According to Unna's 'Abtropfung theory' (trickling down theory), nevus cells of an epidermal origin drop off into the dermis and there they form clusters or nests. This phenomenon is commonly seen when primary melanomas invade into the dermis during the vertical growth phase14. In our case, there were focal atypical melanocytes arranged in the basal layer of the epidermis without dropping off into the dermis. These findings were different from the junctional activity seen in melanomas during the vertical growth phase, and our patient's lesions showed the following characteristics: 1) atypical melanocytes were arranged as single cells that did not form clusters or nests; 2) the findings were focal without pagetoid spread of the malignant tumor cells into the upper layer of the epidermis.
The pathogenesis of malignant blue nevi is not well known. Tumor cells incubated with DOPA have been found to have strongly positive responses, demonstrating that the tumor cells are melanocytes in origin. A tyrosine kinase receptor encoded by the c-kit gene (CD117) has been reported to be important in the proliferation of melanocytes15,16. The expression of the c-kit gene is strong in normal junctional melanocytes and in the junctional component of compound nevi. The level of c-kit is decreased in the dermal component of nevi16. The benign or in-situ components of malignant melanomas are positive for c-kit and they are negative within the invasive part, and the c-kit expression is lost during progression13,16-18. Zyrek-Betts et al.13 reported a patient with a malignant blue nevus and lymph node metastases with CD117 (c-kit) immunohistochemical staining. CD117 staining of the cellular blue nevi component was strongly positive and this was decreased in the dermal component of the invasive melanoma. However, it showed up-regulation in the metastatic lymph nodes, which is unlike the prior reports that have shown a decreased c-kit expression in the metastatic melanoma16-18. Our patient presented with similar findings that were consistent with the case reported by Zyrek-Betts et al.13. These findings suggest that malignant blue nevi are different from other melanomas in their biological behavior.
Our case is unique with regard to the histological features of malignant melanoma that presumably developed in the blue nevus of the lip. The lesion met all the criteria with high grade atypia, spontaneous tumor necrosis (shown as ulcer clinically) and frequent mitoses. Malignant blue nevi might develop from blue or cellular blue nevi, a giant congenital nevus or in a nevus of Ota, or it may be malignant from the start. In our case, we assumed that the blue nevus was malignantly transformed because our patient had a stable pigmented macule on the lip without any changes in shape, size and color for 3 years. The malignant changes, including ulceration and irregular borders, developed over a short period of time. Furthermore, the c-kit expression was decreased in the deep invasive part of the lesion, as opposed to the superficial and peripheral components of the malignant blue nevus. These findings support the suggestion that the c-kit expression is lost during malignant progression17,18.
The prognosis for a malignant blue nevus has varied for both metastasis and survival in different series; this tumor is considered an aggressive lesion with a metastasis rate of up to 83% and a mortality rate of 67~73%19,20. Although there are discrepancies with regard to the rates of metastasis depending on the study, it is clear that this lesion has a poor prognosis.
Figures and Tables
Fig. 1
(A) Bluish black macules (arrow head) with irregular borders and (B) ulcerative nodules (arrow) localized on the upper lip with the development of satellite lesions.
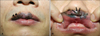
Fig. 2
The histopathological findings of the bluish black macule showed pigmented spindle-shaped and dendritic melanocytes among thickened collagen bundles from the reticular dermis which were close to the epidermis (H&E, ×100).
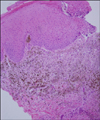
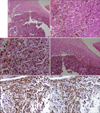
Fig. 3
(A) The histopathologic findings of the bluish black ulcerative nodule showed atypical epithelioid tumor cells and scattered dark pigmented spindle-shaped cells in the dermis. The findings shown include (B) pleomorphic epothelioid tumor cells intermingled with pigmented spindle shaped cells, and (C) necrotic tumor cells (arrow) and mitosis (arrow head). (D) Focal atypical large melanocytes were arranged as single cells on the basal layer of the epidermis without pagetoid spread. The immunohistochemical stains were positive for HMB45 (E) and S-100 (F) (A: H&E, ×100, B: H&E, ×200, C: H&E, ×400, D: H&E, ×200, E: HMB45, ABC method, ×200, F: S-100, ABC method, ×200).
References
2. Granter SR, McKee PH, Calonje E, Mihm MC Jr, Busam K. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called 'malignant blue nevus'. Am J Surg Pathol. 2001. 25:316–323.

3. Meleti M, Mooi WJ, Casparie MK, van der Waal I. Melanocytic nevi of the oral mucosa - no evidence of increased risk for oral malignant melanoma: an analysis of 119 cases. Oral Oncol. 2007. 43:976–981.

4. Buchner A, Leider AS, Merrell PW, Carpenter WM. Melanocytic nevi of the oral mucosa: a clinicopathologic study of 130 cases from northern California. J Oral Pathol Med. 1990. 19:197–201.

5. Scotto J, Fraumeni JF Jr, Lee JA. Melanomas of the eye and other noncutaneous sites: epidemiologic aspects. J Natl Cancer Inst. 1976. 56:489–491.

6. Saida T, Kawachi S, Takata M, Kurita H, Kurashina K, Kageshita T, et al. Histopathological characteristics of malignant melanoma affecting mucous membranes: a unifying concept of histogenesis. Pathology. 2004. 36:404–413.

7. Hu W, Nelson JE, Mohney CA, Willen MD. Malignant melanoma arising in a pregnant African American woman with a congenital blue nevus. Dermatol Surg. 2004. 30:1530–1532.

8. Pathy AL, Helm TN, Elston D, Bergfeld WF, Tuthill RJ. Malignant melanoma arising in a blue nevus with features of pilar neurocristic hamartoma. J Cutan Pathol. 1993. 20:459–464.

9. Hagiwara T, Kaku T, Kobayashi H, Hirakawa T, Nakano H. Coexisting vulvar malignant melanoma and blue nevus of the cervix. Gynecol Oncol. 2005. 99:519–520.

10. Barnhill RL, Argenyi Z, Berwick M, Duray PH, Erickson L, Guitart J, et al. Atypical cellular blue nevi (cellular blue nevi with atypical features): lack of consensus for diagnosis and distinction from cellular blue nevi and malignant melanoma ("malignant blue nevus"). Am J Surg Pathol. 2008. 32:36–44.

11. Mones JM, Ackerman AB. "Atypical" blue nevus, "malignant" blue nevus, and "metastasizing" blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004. 26:407–430.

12. Elder DE, Elenitsas R, Murphy GF, Xu X. Elder DE, Johnson BL, Elenitsas R, editors. Benign pigmented lesions and malignant melanoma. Lever's histopathology of the skin. 2009. 10th ed. Philadelphia: Lippincott Williams & Wilkins;701–704.
13. Zyrek-Betts J, Micale M, Lineen A, Chaudhuri PK, Keil S, Xue J, et al. Malignant blue nevus with lymph node metastases. J Cutan Pathol. 2008. 35:651–657.

14. Gontier E, Cario-Andre M, Vergnes P, Bizik J, Surleve-Bazeille JE, Taieb A. The 'Abtropfung phenomenon' revisited: dermal nevus cells from congenital nevi cannot activate matrix metalloproteinase 2 (MMP-2). Pigment Cell Res. 2003. 16:366–373.

15. Isabel Zhu Y, Fitzpatrick JE. Expression of c-kit (CD117) in Spitz nevus and malignant melanoma. J Cutan Pathol. 2006. 33:33–37.

16. Natali PG, Nicotra MR, Winkler AB, Cavaliere R, Bigotti A, Ullrich A. Progression of human cutaneous melanoma is associated with loss of expression of c-kit proto-oncogene receptor. Int J Cancer. 1992. 52:197–201.

17. Montone KT, van Belle P, Elenitsas R, Elder DE. Protooncogene c-kit expression in malignant melanoma: protein loss with tumor progression. Mod Pathol. 1997. 10:939–944.
18. Willmore-Payne C, Holden JA, Tripp S, Layfield LJ. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol. 2005. 36:486–493.

20. Aloi F, Pich A, Pippione M. Malignant cellular blue nevus: a clinicopathological study of 6 cases. Dermatology. 1996. 192:36–40.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download