Abstract
Acute generalized exanthematous pustulosis (AGEP) is a clinical reaction pattern that is principally drug induced and this is characterized by acute, nonfollicular sterile pustules on a background of edematous erythema. Hydroxychloroquine (HCQ) has been widely used to treat rheumatic and dermatologic diseases and HCQ has been reported to be an uncommon cause of AGEP. A 38-year-old woman with a 1-year history of dermatomyositis and polyarthralgia was treated with HCQ due to a lack of response to a previous medication. Three weeks after starting HCQ therapy, the pustular skin lesion developed and then this resolved after the HCQ was withdrawn and steroid treatment was started. A similar pustular eruption developed after HCQ was accidentally readministered.
Drug induced cutaneous adverse events are reported in 1~8% of all hospitalized patients who are administered medications1. Acute generalized exanthematous pustulosis (AGEP) is characterized by a sudden onset of multiple non-follicular sterile pustules, fever (>38℃) and leukocytosis with an elevated neutrophil count. Spontaneous resolution usually occurs within 15 days without sequelae. AGEP is induced mostly by drugs2, and especially antibiotics3. Annual incidence of AGEP is estimated to be approximately 1 to 5 cases among one million persons2. Hydroxychloroquine (HCQ, oxyklorin™, Myungmoon Pharm. Co., Korea) has an antimalarial action and this drug is used for the treatment of rheumatic and dermatologic diseases due to its immunosuppressive and anti-inflammatory effects4. HCQ has been described as a rare cause of AGEP in the Korean medical literature5.
A 38-year-old woman with a 1-year history of dermatomyositis and polyarthralgia had been taking prednisolone (PRD) for 1 year. However, the lack of response to corticosteroid led us to withdraw the PRD and then to administer 200 mg of a daily dose of oxyklorin™. Twenty one days after starting oxyklorin™ treatment, she developed generalized erythema and edema, and this was followed by a pruritic pustular eruption. The lesions initially developed on the face and arms and they subsequently spread to the rest of the body (Fig. 1).
Clinical examination revealed numerous erythematous, nonfollicular pustules (<5 mm in diameter) on the whole body, except for the mucomembranes, and diffuse superficial desquamation developed as the lesions evolved. The patient's temperature at presentation ranged from 36.2℃ to 37.5℃. The patient had mild fever with leukocytosis (16.70×109/L, N: 4.8-10.8×109/L), an elevated neutrophil count (77.4%, N: 50~75%) and a high erythrocyte sedimentation rate (42 mm/h, N: 20~27 mm/h). Other routine laboratory findings were in the normal ranges.
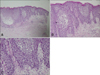
Pathologic findings of the pustular lesion revealed spongiform intraepidermal pustules and perivascular infiltrates containing neutrophils, lymphocytes and eosinophils in the upper dermis (Fig. 2). Based on these clinical/histopathological findings, she was diagnosed with HCQ-induced AGEP.
Oxyklorin™ was withdrawn on the seventh day after the onset of the eruption. She was given a daily dose of intravenous methyprednisolone 125 mg, followed by oral PRD, and the skin lesion resolved completely. Three months later, oxyklorin™ was restarted by a rheumatologist for the treatment of dermatomyositis, and a similar skin eruption developed again 14 days later. The eruption may have been caused by the HCQ or the other ingredients of oxyklorin™ such as glucose, gelatin, talc, magnesium stearate, carnauba wax, castor oil, hydroxypropyl methylcellulose 2910, precipitated calcium carbonate and corn starch. We recommended a patch test and oral provocation test with HCQ and the ingredients of oxyklorin™. But she refused patch test and oral provocation test. We confirmed this was a case a HCQ-induced AGEP by accidental oral provocation.
In 1968, Baker and Ryan6 described 104 cases of pustular psoriasis and five of these patients, who were without a previous history of psoriasis, the pustular eruption was an acute, short course and did not recur. Drugs and/or infection were suspected as the triggering factor. In 1980, Beylot et al.7 introduced the term pustuloses exanthé matiques aiguës généralisées, and it is translated as acute generalized exanthematous pustulosis. In 2001, Sidoroff et al.2 proposed the diagnosis criteria for AGEP and this included (1) a sudden onset of an eruption of numerous, small (<5-mm), non-follicular pustules, (2) a fever above 38℃, (3) neutrophilia with or without mild eosinophilia, (4) histologic evidence of subcorneal or intraepidermal pustules and (5) acute evolution with spontaneous resolution in less than 15 days.
AGEP is induced by drugs in more than 90% of the cases2. Antibiotics such as aminopenicillins, macrolides, ceftriaxone, clindamycin and levofloxacin are the most common triggering agents, while sulfonamides are not. Other drugs, including calcium channel blockers, anticonvulsants, NSAIDs, antiulcer drugs, corticosteroids and HCQ have also been reported in several cases8. Viral infections and mercury hypersensitivity are less common causes9.
Differential diagnosis of AGEP includes various other pustular eruptions such as pustular psoriasis (von Zumbusch type), subcorneal pustular dermatosis (Sneddon-Wilkinson disease), IgA pemphigus, drug rash with eosinophilia and systemic symptoms (DRESS) and toxic epidermal necrolysis (TEN)2,3.
Histologically, AGEP is characterized by subcorneal or superficial intraepidermal pustules and a mild spongiform change at the margins of the pustules. The papillary dermis is usually edematous, and perivascular neutrophils or eosinophils infiltrate are shown in the upper dermis and the presence of necrotic keratinocytes in the epidermis is seen10.
HCQ is a 2-[{4-[(7-chloroquinolin-4-yl)amino]pentyl}(ethyl) amino]ethanol sulfate and it was first used as an antimalarial drug10. It has immunosuppressive and anti-inflammatory effects10, and so it is useful for treating systemic lupus erythematosus, rheumatoid arthritis, Sjögren's syndrome, juvenile chronic arthritis, hepatic sarcoidosis, atrophodermia of Pasini-Pierini and polymorphous light eruption that have not satisfactorily responded to other drugs11. But the mechanism of action of HCQ is unknown. The adverse events of AGEP due to HCQ are divided into two groups: the overdose group and the hypersensitive group. In accidental overdose group, 4-aminoquinoline compounds are very rapidly and completely absorbed, and symptoms may occur within 30 minutes after ingestion. These consist of headache, drowsiness, visual disturbances, cardiovascular collapse and convulsions, followed by sudden and early respiratory and cardiac arrest. Adverse effects in the hypersensitive group are uncommon. Cutaneous side effects of HCQ in the hypersensitivity group include bleaching of hair, alopecia, pruritus, skin and mucosal pigmentation, photosensitivity, exacerbation of psoriasis and skin eruptions (urticarial, morbilliform, lichenoid, maculopapular, purpuric, erythema annulare centrifugum, Stevens-Johnson syndrome, AGEP and exfoliative dermatitis)11.
A standard oral provocation test is a sensitive diagnostic tool, and it may provide an early confirmatory diagnosis of drug induced skin eruption, include AGEP. In this case, initial drug dose for an oral provocation test may be started at 100 mg (half of the therapeutic dosage). If there are no eruptions after administration of initial drug dose, an additional dose of 100 mg may be given after an appropriate time interval12. Because this patient manifesting the same eruption after 15 days of taking oxyklorin™ medication, the time interval for our patient between the different drug dosages was at least 2~3 weeks. Yet she refused an oral provocation test with HCQ and the ingredients of oxyklorin™.
We report here on a rare case of AGEP due to HCQ, and this was confirmed by accidental oral provocation.
Figures and Tables
References
1. Arroyo MP, Heller P, Pomeranz MK. Generalized pustules in a healthy woman. J Drugs Dermatol. 2002. 1:63–65.
2. Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001. 28:113–119.

3. Paradisi A, Bugatti L, Sisto T, Filosa G, Amerio PL, Capizzi R. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: three cases and a review of the literature. Clin Ther. 2008. 30:930–940.

5. Choi JH, Sim HS, Jung Y, Lee SK. A case of acute generalized exanthematous pustulosis induced by hydroxychloroquine. Korean J Dermatol. 2008. 46:138–140.
6. Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol. 1968. 80:771–793.
7. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases). Ann Dermatol Venereol. 1980. 107:37–48.
8. Gebhardt M, Lustig A, Bocker T, Wollina U. Acute generalized exanthematous pustulosis (AGEP): manifestation of drug allergy to propicillin. Contact Dermatitis. 1995. 33:204–205.

9. Braun-Falco O, Luderschmidt C, Maciejewski W, Scherer R. Generalized acute pustulosis. An unusual presentation of leukocytoclastic vasculitis. Hautarzt. 1978. 29:371–377.
10. Burrows NP, Russell Jones RR. Pustular drug eruptions: a histopathological spectrum. Histopathology. 1993. 22:569–573.

11. Plaquenil (hydroxychloroquine sulfate) tablets. Adverse reactions [Internet]. 2006 Oct 5. Bridgewater (NJ): Sanofi;Available from:
http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/009768s041lbl.pdf.
12. Das J, Mandal AC. A study of drug eruptions by provocative tests. Indian J Dermatol Venereol Leprol. 2001. 67:238–239.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download