Abstract
Background
Ultraviolet (UV) radiation has been used for decades to treat a variety of skin diseases. UVA1 was used initially as an effective treatment for acute exacerbated atopic dermatitis. Since then, UVA1 has been attempted for recalcitrant skin diseases.
Objective
This study examined the efficacy of UVA1 phototherapy in three recalcitrant skin diseases.
Methods
This retrospective study reviewed the efficacy and follow-up of 26 patients with atopic dermatitis (AD), mycosis fungoides (MF) and localized scleroderma (LS). SUPUVASUN 3000 (Mutzhas Co., Munich, Germany) and SELLAMED 3000 (Sellas Medizinische Gerate GmbH, Gevelsberg, Germany) were the UVA1 equipment used. Irradiation was performed in accordance with the disease. Low-dose (20 J/cm2), medium-dose (65 J/cm2) and high-dose regimens (100 J/cm2) of UVA1 therapy were employed. The frequency of the therapy ranged from 3 to 5 times weekly. The therapeutic effectiveness was assessed according to the clinical examination before and after the last treatment.
Results
In patients with AD, complete and partial remission was achieved in four (80%) and one (20%) patient, respectively. In patients with MF, complete and partial remission was observed in thirteen (86.7%) and two (13.3%) patients, respectively. In patients with LS, complete and partial remission was observed in three (50%) and three (50%) patients, respectively.
Ultraviolet (UV) radiation has been used for decades to treat a range of skin diseases. UVA1 therapy was developed in the early 1980s and found to induce T-cell apoptosis by mediating singlet oxygen damage1. In addition, it may increase collagenase (matrix metalloproteinase-1) expression2, and decrease the interferon (IFN)-γ level3, the number of Langerhans cells and mast cells in the dermis4. The therapeutic effect of UVA1 is related to the long-wavelengths that penetrate the dermis more deeply than UVB and PUVA5.
In early 1990s, UVA1 therapy was used initially as an effective novel treatment modality for acute exacerbated atopic dermatitis6-8. Since then, UVA1 phototherapy has been attempted for recalcitrant skin diseases with a T-cell abnormality and sclerotic conditions. This study examined the efficacy of UVA1 phototherapy in patients with atopic dermatitis, mycosis fungoides and localized scleroderma.
A total of 26 patients were enrolled in this study after providing informed written consent. All patients were collected from the Department of Dermatology, Kosin University College of Medicine, Busan, South Korea, from June 2002 to July 2008. This retrospective clinical study included the patient's age, gender, diagnosis, duration of disease, previous treatment, UVA1 dose, frequency of therapy, cumulative UVA1 dose, responses to treatment, side effects, and follow-up. The conditions examined included atopic dermatitis (AD; 5 patients), mycosis fungoides (MF; 15 patients) and localized scleroderma (LS; 6 patients). Patients who were pregnant or lactating, had a history of photosensitive dermatitis and were currently using photosensitizing drugs were excluded.
The patients were treated with low-dose (20 J/cm2), medium-dose (65 J/cm2) and high-dose (100 J/cm2) UVA1 therapy. In AD, a high-dose regimen (100 J/cm2) of UVA1 was used. In MF, a low, medium and high-dose regimen (20 J/cm, 65 J/cm2 and 100 J/cm2) of UVA1 was used. In LS, a low and high-dose regimen of UVA1 (20 J/cm2 and 100 J/cm2) was used. The dose and frequency of UVA1 were determined from the literature. Low-dose (20 J/cm2) UVA1 was delivered by SUPUVASUN 3000 (Mutzhas Co., Munich, Germany) and the medium-dose (65 J/cm2) and high-dose (100 J/cm2) UVA1 therapy was delivered by SELLAMED 3000 (Sellas Medizinische Gerate GmbH, Gevelsberg, Germany). The main wavelengths ranged from 340 nm to 440 nm. The irradiation intensity of SUPUVASUN 3000 and SELLAMED 3000 was 27 and 70 mW/cm2 at a distance of 30 cm, respectively. The UVA1 irradiance was measured using an IL 1700 photometer (International Light, Newburyport, MA, USA). The frequency of therapy ranged from 3 to 5 times weekly.
The therapeutic effectiveness was assessed by the same dermatologists before and after UVA1 therapy, and was graded as complete, partial remission or no response. In recalcitrant skin diseases, complete and partial remission were assessed above and below 95% clinical clearing, respectively. In addition, the therapeutic effectiveness of AD was measured using the SCORAD index.
Five patients with atopic dermatitis received 10~15 sessions (mean, 11.2 sessions) of high dose (100 J/cm2) UVA1 therapy (Fig. 1). The cumulative UVA1 doses were 1,000~1,500 J/cm2 (mean, 1,120 J/cm2) (Table 1). Four and 1 of the 5 patients showed complete remission and partial improvement, respectively (Table 2). UVA1 phototherapy led to a 69.9% decrease in the median SCORAD score after 2 weeks. No serious side effects were observed except for some hyperpigmentation.
A total of 15 patients with MF were treated with UVA1 (Table 1).
Three patients who had received low-dose UVA1 (20 J/cm2) for 2~3 times a week showed complete remission. None of these patients relapsed after a mean time of 49.3 months.
Eight patients received medium-dose UVA1 (65 J/cm2) daily or 2~3 times a week (Fig. 2). Of them, complete and partial remission was achieved in seven (87.5%) and one (12.5%) patient, respectively. One of the 7 patients with complete remission recurred after 30 months. The condition was completely cleared with oral acitretin (10 mg/day), topical keratolytics and UVA1 therapy performed 19 times. The patient who recurred achieved complete remission after 14 irradiations of UVA1 at a dose of 65 J/cm2.
Four patients received high-dose UVA1 (100 J/cm2) daily or 2~3 times a week (Fig. 3). Three and 1 patient showed complete remission and partial improvement, respectively. The skin lesions in 1 of the 3 patients showing complete remission recurred. The patient showed complete remission after 39 UVA1 treatments but the skin lesions recurred after 6 months. The patient was treated again with 100 J/cm2 UVA1. Complete remission was achieved after 11 UVA1 treatments.
Among the 15 patients with MF, complete and partial remission was observed in 13 and 2 patients, respectively. In low-dose UVA1 therapy, complete remission was observed in all 3 patients after a mean number of 16.6 UVA1-treatments. In medium-dose UVA1 therapy, complete remission was observed in 7 patients after a mean number of 25.3 UVA1-irradiations and 1 patient showed partial improvement. In high-dose UVA1 therapy, complete remission was observed in 3 patients after a mean number of 26.3 UVA1 treatments and 1 patient showed partial improvement (Table 3).
During the follow-up (ranges from 1 to 68 months), 13 of the 15 patients achieved a complete remission. Only 2 patients relapsed but responded faster to a second course of UVA1 therapy. No serious side effects were observed except for some hyperpigmentation.
Six patients with localized scleroderma consisted of 5 patients with morphea and 1 patient with linear scleroderma (en coup de sabre). All 6 patients received 2~3 times a week. Complete and partial remission was observed in three (50%) and three (50%) patients, respectively) (Table 4).
Two patients showed a complete remission after high-dose UVA1 (Fig. 4). One patient with complete remission received 67 UVA1 sessions at 3~5 times weekly over a 9 week periods, resulting in a cumulative dose of 6,700 J/cm2. Six months after the cessation of treatment, the patient had a new lesion near the first treatment site. The patient achieved complete remission after 16 doses of high-dose UVA1.
One patient showed a complete remission after low-dose UVA1. The patient received 39 sessions of UVA1 twice weekly for 6 weeks, resulting in a cumulative dose of 780 J/cm2. The patient did not relapse during an 84 month follow-up. No serious side effects were observed except for some hyperpigmentation.
UVA1 has been used as an effective treatment modality for patients with several recalcitrant skin diseases. The mechanisms of UVA1 include T-cell apoptosis1, collagenase (matrix metalloproteinase-1) expression2, down regulation of interferon (IFN)-γ3 and a decreased number of Langerhans cells and mast cells in the dermis4. UVA1 penetrates the dermis deeply and has the ability to affect intradermal T-cells, Langerhans cells and mast cells, whereas UVB and PUVA are absorbed mainly by the epidermis and upper dermis5.
Immediate apoptosis or pre-programmed cell death, which is not dependent on protein synthesis, is mediated by the generation of singlet oxygen. Delayed apoptosis, which can be caused by UVB and PUVA, is a programmed cell death that requires protein synthesis9-11. In PUVA therapy, psoralens bind to the DNA molecules and subsequent UVA irradiation causes DNA damage. UVA1 therapy damages the mitochondrial membrane through the production of singlet oxygen10,12. UVA1 can have rapid therapeutic efficacy because it involves two apoptotic pathways.
T-cell apoptosis is related to the treatment of atopic dermatitis (AD), mycosis fungoides (MF) and localized scleroderma (LS). In AD, UVA1 irradiation induces a decrease in IFN-γ, intercellular adhesion molecule (ICAM)-1 and induction of interleukin (IL)-10 in lesional skin3. The decrease in IFN-γ is caused by an indirect mechanism, where anti-inflammatory IL-10 suppresses IFN-γ production by T-helper 1 cells13. In addition, UVA1 irradiation reduces the number of Langerhans cells and mast cells4.
A UVA1 treatment for the management of patients with AD was initially attempted in patients with acute, severe exacerbation. In the early 1990s, Krutmann et al.7,8 reported that initially, high-dose UVA1 phototherapy is effective for patients with acute exacerbated AD. Since then, the optimal dose for therapeutic efficacy has been evaluated in many studies14-17. Although a high-dose regimen for patients with AD is used widely, some studies have reported that medium-dose UVA1 phototherapy is effective against acute exacerbated AD14,16. However, the efficacy of medium-dose UVA1 was short-term and the symptoms recurred within 3-months16. In another study, it was shown that a high and medium-dose UVA1 was more effective than a low-dose regimen17. According to studies of the dosing regimen of UVA1, many studies demonstrated that high and medium-dose UVA1 were superior to a low-dose regimen. With the exception of atopic dermatitis, it is unclear if the UVA1 dose-dependent response for numerous UVA1 responsive diseases is due to a cumulative or single UVA1 dose. Although UVA1 therapy is effective in patients with acute exacerbated AD, relapses are common. Broadband UVB therapy, broadband UVA therapy, UVA/UVB therapy, PUVA, narrowband UVB and low-dose UVA1 therapy are used as maintenance treatments for chronic AD. Several studies demonstrated that NBUVB therapy is effective against chronic AD18,19. In the present study, high-dose UVA1 phototherapy was performed on patients with acute exacerbated AD. In patients showing complete remission, the mean number of UVA1 irradiation treatments and mean cumulative UVA1 dose was 11.5 and 1,150 J/cm2 (1,000~1,500 J/cm2), respectively. It is believed that 10 or more exposures to high-dose UVA1 may be appropriate for achieving complete remission in AD patients.
However, there is some inconvenience with UVA1 irradiation. The UVA1 equipment is not a cabinet type, so its irradiation field is limited. Multiple of UVA1 treatments are necessary for patients with generalized skin lesions.
Besides T-cell apoptosis, UVA1 induces the release of IFN-γ at 4 h after UVA1 irradiation. IFN-γ stimulates the production of tumor necrosis factor-α (TNF-α), which kills neoplastic cells. Therefore, neoplastic disease, such as MF, can be treated effectively and rapidly11.
MF shows a good treatment response in the early patch or plaque stages. Since MF has a long disease duration, MF patients require a long-term and safe treatment. In the early stages, the treatment methods of MF include topical application of corticosteroids, nitrogen mustard and bexarotene, PUVA, UVB, UVA1 and spot radiotherapy. If the disease progresses, systemic retinoids, such as acitretin and bexarotene, extracorporeal photochemotheapy, interferon-alpha, and denileukin diftitox (Ontak®), are needed20. UVA1 phototherapy is effective, gives a fast response and safe in early and advanced stages. Several studies have reported the good therapeutic efficacy of UVA1 phototherapy. Plettenberg et al.21 reported that UVA1 phototherapy for early MF is more effective and has a more rapid response than PUVA. Zane et al.22 reported that UVA1 irradiation is quite effective against early and advanced MF.
In these results, the UVA1 therapy had excellent therapeutic effectiveness in patients with MF treated. Complete remission was observed after 20 to 30 exposures to medium and high UVA1 doses. Therefore, UVA1 therapy is superior to PUVA for the following reasons: (1) UVA1 irradiation penetrates deeply into the dermis5; (2) UVA1 irradiation induces both an immediate and delayed apoptosis but PUVA therapy can only trigger the latter, which is related to DNA-damaged programmed cell death10. (3) UVA1 does not have the unwanted side effects of psoralen-induced phototoxicity and gastrointestinal reactions. Therefore, UVA1 phototherapy is more effective, has a quicker response and is better tolerated than PUVA in MF patients. Once remission is achieved, maintenance therapy is indicated. PUVA is used most commonly for maintenance treatment. In addition, topical steroid and interferon can also be used.
The mechanisms of UVA1 in the treatment of LS is the up-regulation of collagenase and a decrease in transforming growth factor beta (TGF-β), proinflammatory cytokines (IL-1, 6)2,23,24. In addition, LS can be treated effectively because an increase in the IFN-γ level by UVA1 inhibits collagen production11,25.
LS is a connective tissue disease involving thickening of the skin. The treatment of LS is unsatisfactory. Topical and systemic steroids, antimalarials, phenytoin, colchicine, D-penicillamine, immunosuppressive agents such as azathioprine, chlorambucil, methotrexate, cyclophosphamide, cyclosporine, plasmapheresis, extracorporeal photopheresis are used in LS, but with various degrees of efficacy. Therefore, many studies have investigated the therapeutic effectiveness of phototherapy in the treatment of LS26-30. UV therapy for LS patients was introduced by PUVA in 199426. However, PUVA may have disadvantages, such as gastrointestinal and carcinogenic effects. In 1995, Kerscher et al.31 reported that low-dose UVA1 phototherapy could be used initially in LS. Subsequently, they demonstrated the therapeutic effectiveness of low-dose UVA1 therapy in 20 patients with LS. The patients received 20 J/cm2 UVA1 for 30 sessions with a cumulative dose of 600 J/cm232. In 1997, Stege et al.29 reported that high-dose UVA1 therapy is effective in patients with LS. Ten patients received 130 J/cm2 UVA1 for 30 sessions with a cumulative dose of 3,900 J/cm2. Seven patients received 20 J/cm2 for 30 sessions with a cumulative dose of 600 J/cm2 UVA1. The authors asserted that high-dose UVA1 is superior to low-dose UVA1. In 2003, a controlled study with medium-dose UVA1 (48 J/cm2) was performed in 8 patients. The patients were treated 4 times weekly for 12 weeks and showed an improvement in skin sclerosis33.
In the present study, 3 and 3 patients received low-dose (20 J/cm2) and high-dose (100 J/cm2) UVA1 therapy, respectively. One and 2 patients showing complete remission received low- and high-dose UVA1 phototherapy, respectively. Both low- and high-dose UVA1 phototherapy in these patients with LS were effective. However, the optimal dose of UVA1 was not determined. Therefore, a further study will be needed to confirm its therapeutic efficacy for a single dose and cumulative doses of UVA1.
Although the conclusions from this study are limited by its retrospective design, UVA1 therapy produced good therapeutic effects against AD, MF and LS. To our knowledge, this is the first and largest study on the efficacy of UVA1 phototherapy in dark-skinned peoples. Further prospective studies will be needed to determine the therapeutic effects of UVA1 phototherapy in a range of diseases.
Figures and Tables
Fig. 1
Atopic dermatitis lesions (A) before and (B) after 11 irradiations with 100 J/cm2 UVA1 phototherapy.
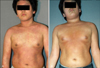
Fig. 2
Solitary mycosis fungoides lesion (A) before and (B) after 15 irradiations with 65 J/cm2 UVA1 phototherapy.
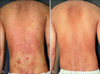
Fig. 3
Mycosis fungoides lesions (A) before and (B) after 22 irradiations with 100 J/cm2 UVA1 phototherapy.
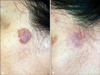
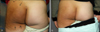
Fig. 4
Localized scleroderma lesion (A) before and (B) after 86 irradiations with 100 J/cm2 UVA1 phototherapy.
References
1. Morita A, Werfel T, Stege H, Ahrens C, Karmann K, Grewe M, et al. Evidence that singlet oxygen-induced human T helper cell apoptosis is the basic mechanism of ultraviolet-A radiation phototherapy. J Exp Med. 1997. 186:1763–1768.

2. Gruss C, Reed JA, Altmeyer P, McNutt NS, Kerscher M. Induction of interstitial collagenase (MMP-1) by UVA-1 phototherapy in morphea fibroblasts. Lancet. 1997. 350:1295–1296.

3. Grewe M, Gyufko K, Schopf E, Krutmann J. Lesional expression of interferon-gamma in atopic eczema. Lancet. 1994. 343:25–26.
4. Grabbe J, Welker P, Humke S, Grewe M, Schopf E, Henz BM, et al. High-dose ultraviolet A1 (UVA1), but not UVA/UVB therapy, decreases IgE-binding cells in lesional skin of patients with atopic eczema. J Invest Dermatol. 1996. 107:419–422.

6. Mutzhas MF, Holzle E, Hofmann C, Plewig G. A new apparatus with high radiation energy between 320-460 nm: physical description and dermatological applications. J Invest Dermatol. 1981. 76:42–47.

7. Krutmann J, Czech W, Diepgen T, Niedner R, Kapp A, Schopf E. High-dose UVA1 therapy in the treatment of patients with atopic dermatitis. J Am Acad Dermatol. 1992. 26:225–230.

8. Krutmann J, Schopf E. High-dose-UVA1 phototherapy: a novel and highly effective approach for the treatment of acute exacerbation of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1992. 176:120–122.
9. Godar DE. Preprogrammed and programmed cell death mechanisms of apoptosis: UV-induced immediate and delayed apoptosis. Photochem Photobiol. 1996. 63:825–830.

10. Godar DE. UVA1 radiation triggers two different final apoptotic pathways. J Invest Dermatol. 1999. 112:3–12.

11. Godar DE, Lucas AD. Ultraviolet-A1 (340-400 nm)-mediated receptor and cytokine changes of transformed lymphocytes. Photodermatol Photoimmunol Photomed. 2005. 21:23–31.

12. Godar DE, Lucas AD. Spectral dependence of UV-induced immediate and delayed apoptosis: the role of membrane and DNA damage. Photochem Photobiol. 1995. 62:108–113.

13. Grewe M, Gyufko K, Krutmann J. Interleukin-10 production by cultured human keratinocytes: regulation by ultraviolet B and ultraviolet A1 radiation. J Invest Dermatol. 1995. 104:3–6.

14. Kowalzick L, Kleinheinz A, Weichenthal M, Neuber K, Kohler I, Grosch J, et al. Low dose versus medium dose UV-A1 treatment in severe atopic eczema. Acta Derm Venereol. 1995. 75:43–45.
15. Krutmann J, Diepgen TL, Luger TA, Grabbe S, Meffert H, Sonnichsen N, et al. High-dose UVA1 therapy for atopic dermatitis: results of a multicenter trial. J Am Acad Dermatol. 1998. 38:589–593.

16. Abeck D, Schmidt T, Fesq H, Strom K, Mempel M, Brockow K, et al. Long-term efficacy of medium-dose UVA1 phototherapy in atopic dermatitis. J Am Acad Dermatol. 2000. 42:254–257.
17. Tzaneva S, Seeber A, Schwaiger M, Honigsmann H, Tanew A. High-dose versus medium-dose UVA1 phototherapy for patients with severe generalized atopic dermatitis. J Am Acad Dermatol. 2001. 45:503–507.

18. Der-Petrossian M, Seeber A, Honigsmann H, Tanew A. Half-side comparison study on the efficacy of 8-methoxypsoralen bath-PUVA versus narrow-band ultraviolet B phototherapy in patients with severe chronic atopic dermatitis. Br J Dermatol. 2000. 142:39–43.

19. Legat FJ, Hofer A, Brabek E, Quehenberger F, Kerl H, Wolf P. Narrowband UV-B vs medium-dose UV-A1 phototherapy in chronic atopic dermatitis. Arch Dermatol. 2003. 139:223–224.

20. Whittaker SJ, Marsden JR, Spittle M, Russell Jones R. Joint British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous T-cell lymphomas. Br J Dermatol. 2003. 149:1095–1107.

21. Plettenberg H, Stege H, Megahed M, Ruzicka T, Hosokawa Y, Tsuji T, et al. Ultraviolet A1 (340-400 nm) phototherapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 1999. 41:47–50.

22. Zane C, Leali C, Airo P, De Panfilis G, Pinton PC. "High-dose" UVA1 therapy of widespread plaque-type, nodular, and erythrodermic mycosis fungoides. J Am Acad Dermatol. 2001. 44:629–633.

23. Matich AJ, Tilbury RN, Jordan TW. Characteristics of the chemiluminescence from the blood plasma of a normal human population. Photochem Photobiol. 1995. 62:550–556.

24. Gambichler T, Skrygan M, Tomi NS, Breuksch S, Altmeyer P, Kreuter A. Significant downregulation of transforming growth factor-beta signal transducers in human skin following ultraviolet-A1 irradiation. Br J Dermatol. 2007. 156:951–956.

25. Gillery P, Serpier H, Polette M, Bellon G, Clavel C, Wegrowski Y, et al. Gamma-interferon inhibits extracellular matrix synthesis and remodeling in collagen lattice cultures of normal and scleroderma skin fibroblasts. Eur J Cell Biol. 1992. 57:244–253.
26. Kerscher M, Volkenandt M, Meurer M, Lehmann P, Plewig G, Rocken M. Treatment of localised scleroderma with PUVA bath photochemotherapy. Lancet. 1994. 343:1233.

27. Kerscher M, Meurer M, Sander C, Volkenandt M, Lehmann P, Plewig G, et al. PUVA bath photochemotherapy for localized scleroderma. Evaluation of 17 consecutive patients. Arch Dermatol. 1996. 132:1280–1282.

28. Morison WL. Psoralen UVA therapy for linear and generalized morphea. J Am Acad Dermatol. 1997. 37:657–659.

29. Stege H, Berneburg M, Humke S, Klammer M, Grewe M, Grether-Beck S, et al. High-dose UVA1 radiation therapy for localized scleroderma. J Am Acad Dermatol. 1997. 36:938–944.

30. Kreuter A, Hyun J, Stucker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. J Am Acad Dermatol. 2006. 54:440–447.

31. Kerscher M, Dirschka T, Volkenandt M. Treatment of localised scleroderma by UVA1 phototherapy. Lancet. 1995. 346:1166.

32. Kerscher M, Volkenandt M, Gruss C, Reuther T, von Kobyletzki G, Freitag M, et al. Low-dose UVA phototherapy for treatment of localized scleroderma. J Am Acad Dermatol. 1998. 38:21–26.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download