Abstract
The cutaneous diseases associated with progesterone are autoimmune progesterone dermatitis, erythema multiforme-like eruption, drug-induced progesterone dermatitis and solar urticaria. Estrogen and progesterone are widely used in oral contraceptives and hormone replacement therapies, and they are rarely known to cause a photosensitive reaction. The mechanism of contraceptive-induced photosensitivity is uncertain. Estrogen, rather than progesterone, in the combined oral contraceptive pill has been most frequently implicated in the induction of photosensitivity. A 32-year-old woman presented with an erythematous patch with an itching sensation on the centrofacial area of a residual vitiligious lesion. She had a history of being previously treated with narrow band UVB for 1 year. Her skin lesions had mostly subsided, but some lesions continued. She underwent an in vitro fertilization-embryo transfer 3 months previously, and she then took synthetic progesterone for 3 weeks starting at the 4th week of pregnancy. She was in good health with neither a family history of photosensitivity nor a personal history of any other drug ingestion or topical agent such as sunscreen in association with the beginning of her lesions. Phototesting revealed her to be markedly photosensitive in the UVB and UVA ranges. The intradermal skin reactions to progesterone combined with irradiation with UVA or UVB were positive. We report here on an unusual case of photosensitivity that was localized in a vitiliginous lesion, and this was associated with the intramuscular injections of synthetic progesterone that she had received during an in vitro fertilization-embryo transfer.
Synthetic progesterone has been used to prevent habitual or threatened abortion. Recently, it is increasingly being used as part of an assisted reproductive technique for the infertility related to luteal insufficiency. While many women complain of worsening acne and water retention during their menstrual cycle, there exists a small number for whom the menstrual cycle is associated with a variety of other skin manifestations1. The cutaneous diseases associated with progesterone are autoimmune progesterone dermatitis, erythema multiforme-like eruption, druginduced progesterone dermatitis and solar urticaria. Among these, a photosensitivity reaction associated with progesterone has rarely been reported2. However, the mechanism of contraceptive-induced photosensitivity is uncertain.
In the depigmented areas, because of the deficiency of melanin, the dose of ultraviolet light reaching the deepest layers of the epidermis and the underlying dermis are much greater than that in the areas where the skin is exposed to light, but the skin is not depigmented3. As a consequence, the only areas of the skin to show any adverse reactions are those that are both depigmented and exposed to sunlight3. As our patient had not suffered from any adverse reaction to sunlight before, it seems almost certain that the reaction on this occasion was precipitated by progesterone, which caused symptoms only in the depigmented areas where the effect of ultraviolet irradiation was sufficient to cause an erythematous reaction.
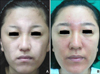
A 32-year-old woman had a past history of undergoing narrow-band UVB phototherapy for about one year for the vitiligo on her face. The vitiliginous lesion had been improved, yet it is still partially remained. She then visited our hospital for multiple erythematous patches and pruritis localized in the vitiliginious lesion (Fig. 1). She had undergone an in vitro fertilization-embryo transfer 3 months previously, and then she took synthetic progesterone for 3 weeks starting at the 4th week of pregnancy. She was in good health with neither a family history of photosensitivity nor a history of any other drug ingestion or use of topical agent such as sunscreen in association with the beginning of the facial lesions.
The differential diagnoses of a photosensitivity reaction secondary to contraceptive pills and polymorphic light eruption were considered. She stopped the contraceptive pills and 1 month later she underwent photopatch testing, and her intradermal skin reactions to progesterone were also assessed. Phototesting revealed her to be markedly photosentive to UVB (MED-UVB 56 mJ/cm2) and UVA (MED-UVA 1 J/cm2), but not visible light (Fig. 2). A photopatch test series irradiated with UVA 0.5 J/cm2 was normal. Anti-nuclear antibody, anti-SSA and anti-SSB were all negative. The urine porphyrin was normal. The serum progesterone (34.6 ng/ml) and estrogen (>1,000 pg/ml) were increased.
Sensitization by exogenous progesterone was suspected and intradermal testing was carried out with utrogestan®, which is composed of micronized progesterone (the principal component) and peanut oil (the excipient). It was used at a dilution of 50 mg/ml of sterile normal saline, and normal sterile saline also served as a control. A gause needle was used to inject 0.1 ml each time. The test was negative. But the intradermal skin reactions to utrogestan irradiated with UVA or UVB were positive (Fig. 3). The hormone medication was withdrawn and she was treated with topical corticosteroid and told to avoid sunlight. After the treatment was initiated, the patient's premenstrual outbreaks of facial lesions steadily decreased in intensity until she no longer had the past symptoms of this condition.
Combined oral contraceptives pills are widely prescribed throughout the world. It is likely that photosensitivity from these pills is a rare problem. There are few reports available on progesterone-induced photosensitivity disorder. Clinically, the nonpapular nature of the eruption and the symptoms of prickling were observed in this current case of contraceptive-induced photosensitivity. When the patient stopped taking the contraceptives, the abnormal phototest reaction reverted to normal4. The mechanism of contraceptive-induced photosensitivity is uncertain. Many investigators have suggested that photosensitivity is due to the hepatotoxicity and the direct effect of the estrogen component of the pills on porphyrin synthesis5. Two types of photosensitivity induced by estrogens, i.e., hepatic porphyria and a polymorphic light eruption-like dermatosis, have been previously observed6. The role of progesterone in photosensitivity has not been previously reported.
Our patient showed photosensitivity to UVA and UVB on the phototest. Clinically, she showed an erythematous patch with an itching sensation on only the centrofacial area of the residual vitiliginous lesion. The intradermal testing of progesterone with UVA and UVB irradiation were positive. Yet the intradermal skin test with progesterone was negative. Unfortunately, we could not perform the skin test with the excipient (peanut oil) due to a lack of corporation. She didn't show any previous history of a cyclic skin rash in the luteal phase of her menstrual cycle. So, we diagnosed her as suffering with progesterone-induced photosensitivity in the vitiliginous area during pregnancy rather than progesterone-induced dermatitis. The low dose of UVA (1 J/cm2) and UVB (5 mJ/cm2) and the negative intradermal skin test with progesterone makes the diagnosis of phototoxicity likely rather than the diagnosis of photoallergy.
It is interesting that two critical factors in the photosensitivity of this patient must be considered. First is the hormonal effect of photosensitivity during pregnancy. The patient started the intramuscular injections of synthetic progesterone at 4 weeks of pregnancy. Physiologically, before the luteoplacental shift, the corpus luteum of the pregnant woman continues to produce progesterone and estrogen during the first 8 to 9 weeks and hormone β-HCG is released from the developing placenta7. The corpus luteum then becomes redundant and the steroid hormone production is taken over by the placenta. The patient took an intra-musculature injection of synthetic progesterone for 3 weeks. This period increased the estrogen production of the corpus luteum. The photosensitivity of this patient may be associated with the synthetic progesterone combined with the estrogen from the corpus luteum. Second was a difference of photoprotection between the vitiliginous area and the adjacent skin. In the vitiliginous areas, because of the deficiency of melanin, the dose of ultraviolet light reaching the deepest layers of the epidermis and the underlying dermis was much greater than that in the normal skin. So, the vitiligo patient complained that the depigmented area suffered from photosensitivity, although the remaining skin reacted normally to sunlight. We think that the patient had a subclinical degree of photosensitivity to sunlight, and this was caused by the progesterone.
We report here on a case of photosensitivity that was localized in a vitiliginous facial lesion, and this photosensitivity was associated with the intramuscular injections of synthetic progesterone she had received during an in vitro fertilization-embryo transfer.
Figures and Tables
References
1. Baptist AP, Baldwin JL. Autoimmune progesterone dermatitis in a patient with endometriosis: case report and review of the literature. Clin Mol Allergy. 2004. 2:10.
2. Cooper SM, George S. Photosensitivity reaction associated with use of the combined oral contraceptive. Br J Dermatol. 2001. 144:641–642.

4. Ro KW, Jung JG, Shim SD, Kim MH, Cinn YW. A case of photosensitivity induced by the hormonal replacement agents containing estrogen and progesterone. Korean J Dermatol. 2005. 43:1098–1101.
5. Sixel-Dietrich F, Doss M. Hereditary uroporphyrinogen-decarboxylase deficiency predisposing porphyria cutanea tarda (chronic hepatic porphyria) in females after oral contraceptive medication. Arch Dermatol Res. 1985. 278:13–16.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download