Abstract
Mycobacterium abscessus (M. abscessus) is an acid-fast bacillus that's classified as a pathogenic "rapid growing" nontuberculous mycobacteria. It is an uncommon cause of human disease, but it can cause skin and soft tissue infection after skin injury following inoculation, minor trauma and surgery. The single most important factor for determining the course and prognosis of a M. abscessus infection is the underlying immune status of the host. We report here on a 71-year-old female who presented with multiple painful erythematous cutaneous nodules on her left forearm. She had diabetes mellitus and had taken oral steroid by herself for two years because of her osteoarthritis. Histologically, granulomas and inflammatory cell infiltration were observed and M. abscessus was identified via the mycobacterial culture. We performed curettage and drainage, followed by 6 months of oral clarithromycin and the patient's disease completely healed.
Mycobacterium abscessus (M. abscessus) is one of the rapidly growing mycobacterial species and it is found in soil, dust and water. M. abscessus was first described by Moore and Frerichs in 1953, and these organisms are ubiquitous in the environment1. It is an uncommon cause of human diseases, but it can cause skin and soft tissue infection after skin injury following inoculation, minor trauma or surgery1,2. The single most important factor for determining the course and prognosis of a M. abscessus infection is the underlying immune status of the host3. Several cases of skin and soft tissue infection caused by M. abscessus have been reported in Korea4-8.
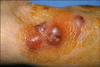
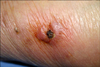
A 71-year-old woman visited our hospital with a week history of multiple painful erythematous swollen nodules and plaques on the left forearm (Fig. 1). She was a diabetes patient and had taken an injection of unknown material for managing the chronic pain of the affected arm. In addition, she had taken oral steroid by herself for about two years for treating her osteoarthritis.
The laboratory examinations to identify bacteria or fungus in the pus specimen from the patient revealed negative results. Acid fast bacilli (AFB) staining and polymerase chain reaction (PCR) analysis for mycobacteria (M. tuberculosis and mycobacterium other than tuberculosis) were also performed on the pus specimen and the results were negative.
Culture from the pus specimen on 3% Ogawa media showed yellowish colonies. The colonies were identified as M. abscessus by polymerase chain reaction-restriction fragment length polymorphism analysis (PCR-RFLP, PRA) with using the novel region of the rpo B gene obtained from the Korean Institute of Tuberculosis, Seoul, Korea (Fig. 2). The mycobacterial species showed resistance to isoniazid, rifampicin, streptomycin, ethombutol, kanamycin, capreomycin, prothionamaide, cycloserine, pyrazinamide and ofloxacin.
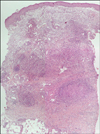
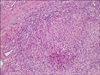
The histological findings showed polymorphonuclear microabscesses and granulomatous inflammation in the dermis and subcutaneous fat tissue (Fig. 3, 4). No acid fast bacilli were observed by AFB staining. Gram staining and PAS staining were also negative. Culture and PCR analysis for mycobacteria were also performed on the tissue specimen and the results were negative.
Curettage and drainage, followed by 6 months of oral clarithromycin administration induced the complete healing of the lesions. After a 6 month remission period, similar skin lesions developed on the left forearm (Fig. 5). The patient had received acupuncture procedures on the left forearm to control pain of the arm. It was estimated that the skin lesions appeared 2 weeks after the initial acupuncture session. Any mycologic study from the pus specimen of new skin lesions revealed negative result. Skin biopsy showed granulomatous inflammation, but AFB staining, culture and PCR analysis from the tissue specimen showed negative results for M. abscessus. Considering that the clinical and pathologic features of the new infection were similar to those of the previous infection, we assumed that the lesions were caused by the recurrence or reinfection of M. abscessus and we administered oral clarithromycin for 3 months. The skin lesions showed improvement after a month of treatment.
The nomenclature of rapidly growing atypical mycobacteria has recently changed with the advent of biochemical techniques, which allow species to be more accurately identified. In the past they were simply identified as belonging to the 'M. fortuitum complex'. Now, with using DNA homology studies, four separate species have been identified as M. fortuitum, M. peregrinum, M. chelonae and M. abscessus. Each of them is associated with different disease syndromes, and each of them has a distinct anti-microbial susceptibility pattern9.
M. abscessus was first described by Moore and Frerichs1 in 1953, and this was isolated from a woman with chronic osteoarthritis. She developed gluteal abscesses that yielded the mycobacterium, and they termed the species 'abscessus'. Among the rapidly growing mycobacterial species, M. abscessus is known to be a major cause of skin infections. It is a cause of nosocomial infections, postinjection abscesses and wound infections following surgeries2,8,10. Inman et al11 reported an outbreak in 1969 amongst 12 patients who were being treated at an ENT clinic where all had received contaminated histamine injections. Galil et al12 reported on a multistate outbreak of postinjection abscesses that were associated with the use of an unlicenced injectable product that was contaminated by mycobacterium. Multiple cutaneous abscesses have been reported in patients who are immunosuppressed, such as renal transplant recipients3. We believe the source of M. abscessus infection in our patient was the injection of unapproved medicine that she had taken on the arm. Her diabetes and chronic oral steroid medication might have played an important role in the manifestation and recurrence of her M. abscessus infection.
Cutaneous infections with rapidly growing mycobacteria can manifest in a variety of ways, including ulcerations, abscesses, draining sinuses or nodules2,8. Histologically, rapidly growing mycobacteria are characterized by a dimorphic inflammatory response that consists of polymorphonuclear microabscesses in the dermis and subcutaneous tissues, along with epithelioid granulomas and giant cells13.
The diagnosis of cutaneous tuberculosis is usually difficult owing to false-negative cultures and negative direct-smear detection. The rapid detection and identification of M. tuberculosis or mycobacterium other than tuberculosis (MOTT) by PCR would provide a prompt differential diagnosis and guide proper management depending on the specific diagnosis. Yet there have been several reports on cases of false-negative results due to the absence of a multicopy gene in certain strains of mycobacteria, and false-positive results due to amplification from MOTT have been reported. M. abscessus and other mycobacterial species have recently been identified by employing more sensitive detection methods such as PCR-RFLP analysis6,8.
The treatment of infection with rapidly growing mycobacteria depends on the extent of disease and the underlying immune status of the host3. M. abscessus is usually resistant to conventional anti-tuberculous drugs, and it is generally susceptible to parenteral therapy with amikacin, cefoxitin and imipenem, and to oral medication with clarithromycin2,3. Because treatment usually extends for 3 to 6 months, oral clarithromycin is considered to be the first-line agent for localized M. abscessus infection8. It is not clear for how long treatment should be continued, but as with other mycobacterial infections, many authors feel that 6 months of treatment should be given, and especially for immunocompromised patients3. Our treatment started with 2nd generation cephalosporin and surgical drainage before the identification of the causative agent, but the lesion showed no improvement and then it became rather aggravated. Oral clarithromycin was administrated after we identified M. abscessus, and the skin lesion showed improvement after a month of treatment.
Although rare, an atypical mycobacterial infection should be considered for the case of a local cutaneous infection that's resistant to antibiotic therapy, and especially if this is seen on an immunocompromised patient.
Figures and Tables
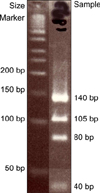 | Fig. 2Polymerlase chain reaction-restriction fragment length polymorphism analysis (PCR-RFLP) shows 105 bp, 80 bp and 40 bp bands that were compatible with Mycobacterium abscessus. |
References
1. Moore M, Frerichs JB. An unusual acid-fast infection of the knee with subcutaneous, abscess-like lesions of the gluteal region; report of a case with a study of the organism, Mycobacterium abscessus, n. sp. J Invest Dermatol. 1953. 20:133–169.

2. Fitzgerald DA, Smith AG, Lees A, Yee L, Cooper N, Harris SC, et al. Cutaneous infection with Mycobacterium abscessus. Br J Dermatol. 1995. 132:800–804.
3. Morris-Jones R, Fletcher C, Morris-Jones S, Brown T, Hilton RM, Hay R. Mycobacterium abscessus: a cutaneous infection in a patient on renal replacement therapy. Clin Exp Dermatol. 2001. 26:415–418.

4. Lee SH, Kim KY, Hong SP, Kim MJ, Yang MH, Seou JT. A Mycobacterium Chelonae Subsp. abscessus wound infection after percutaneous endoscopic gastrostomy. Korean J Med. 1997. 53:842–846.
5. Cho JH, Kim MY, Park YM, Kim HO. A case of cutaneous infection due to Mycobacterium abscessus. Korean J Dermatol. 2004. 42:512–515.
6. Choi YL, Lee KJ, Lee DY, Lee ES. A case of skin infection caused by Mycobacterium abscessus. Korean J Dermatol. 2005. 43:852–855.
7. Kim HS, Park HJ, Lee JY, Cho BK. Twelve cases of cutaneous infection by Mycobacterium abscessus: review on its treatment modality. Korean J Dermatol. 2005. 43:1603–1609.
8. Ryu HJ, Kim WJ, Oh CH, Song HJ. Iatrogenic Mycobacterium abscessus infection associated with acupuncture: clinical manifestations and its treatment. Int J Dermatol. 2005. 44:846–850.

9. Wallace RJ Jr, Tanner D, Brennan PJ, Brown BA. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993. 119:482–486.

10. Brantley JS, Readinger AL, Morris ES. Cutaneous Infection with Mycobacterium abscessus in a child. Pediatr Dermatol. 2006. 23:128–131.

11. Inman PM, Beck A, Brown AE, Stanford JL. Outbreak of injection abscesses due to Mycobacterium abscessus. Arch Dermatol. 1969. 100:141–147.

12. Galil K, Miller LA, Yakrus MA, Wallace RJ Jr, Mosley DG, England B, et al. Abscesses due to mycobacterium abscessus linked to injection of unapproved alternative medication. Emerg Infect Dis. 1999. 5:681–687.

13. Rodriguez G, Ortegon M, Camargo D, Orozco LC. Iatrogenic Mycobacterium abscessus infection: histopathology of 71 patients. Br J Dermatol. 1997. 137:214–218.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download