Abstract
Vitiligo is a common skin disease, but its pathogenesis has not been fully determined, though an autoimmune etiology is considered likely. Kawasaki disease (KD) is an acute multisystem vasculitis of childhood associated with coronary arteriopathy, and is diagnosed based on clinical criteria. Furthermore, vitiligo has been associated with several other diseases, but no report has been issued about the relationship between vitiligo and Kawasaki's disease. The author's report the case of an 8-year-old male child that presented with depigmented lesions, which developed from the desquamative skin lesions of Kawasaki's disease.
Vitiligo is a common skin disease characterized by hypopigmented macules or patches1. Its pathogenesis is has not been elucidated, but an autoimmune mechanism is considered likely2-5. Kawasaki disease (KD) is an acute, immune-related multisystem vasculitis of infants and young children of undetermined etiology6. During the acute disease phase, skin findings are diverse, and during the convalescent phase, desquamative skin lesions tend to appear, especially periungually or perianally7.
Vitiligo is known to be associated with many diseases in adults, such as, alopecia areata, diabetes mellitus, pernicious anemia, Addison's disease, and Hashimoto's thyroiditis8, and in childhood, a strong association with autoimmune thyroiditis has been suggested9.
However, no report is available of the relationship between vitiligo and KD. Herein, we report one case of vitiligo that developed from the skin lesions of KD.
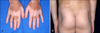
An 8-year-old Korean boy presented with hypopigmented macules and patches on the trunk and upper and lower extremities. Four months previously, he had been admitted to our pediatric cardiology department for a fever which continued for about 7 days, and immediately after admission, skin rashes appeared on his trunk and extremities. His medical history was unremarkable. At physical examination, he was ill-looking and febrile with erythematous skin rashes, which consisted of desquamative patches on the buttocks and on the distal portions of the upper extremities (Fig. 1). His conjunctivae were injected, his tongue showed a strawberry appearance, and his cervical lymph nodes were palpably enlarged. The remainder of the physical examination was unremarkable. Echocardiography revealed left main coronary artery ectasia with mitral regurgitation. KD was diagnosed based on the presence of its typical clinical features. Laboratory analyses on admission demonstrated thrombocytosis (437,000/µl), elevated ESR (30 mm/Hr) and elevated pro-BMP (742.8 pg/ml). Blood chemistry and urinalysis were within normal limits, and blood and conjunctival cultures were negative for bacteria.
When he first presented at our dermatology clinic, hypopigmented macules and patches were noted at sites of previous skin rashes (Fig. 2). He had no familial history of vitiligo. Of the autoimmune antibody studies, an antinuclear antibody (ANA) test showed a 1:100 weekly positive centriole pattern. Histological examinations of a hypopigmented patch from the patient's hand revealed the absence of epidermal melanocytes, and this was confirmed by immunostaining for melan A (Fig. 3). These findings were consistent with a diagnosis of vitiligo. The patient was treated with narrow band ultraviolet B twice weekly, which resulted in a gradual recovery of pigmentation level over 2 months.
Our patient displayed sufficient clinical findings to enable a diagnosis of KD. These criteria are; (1) a fever of more than 5 days duration, (2) skin changes at the extremities, including erythema on palms and soles, (3) polymorphous skin rashes, (4) bilateral conjunctival injections, (5) changes in oral mucosa, reddening of lips, and a 'strawberry' tongue, and (6) cervical lymphadenopathy10. To enable a diagnosis of classic KD, the presence of a fever for more than 5 days and at least 4 of the above five principal criteria are required. As demonstrated by the above criteria, skin changes are important features of KD. Many morphological skin changes have been reported in the literature, such as, scarlatiniform or morbilliform exanthema, generalized erythema, erythema marginatum, erythema multiforme11, psoriatic eruptions7, and palmoplantar pustular eruptions12.
Skin changes in the extremities are known to be distinctive. During the acute phase of KD, erythema of the palms and soles or firm and sometimes painful indurations of hands or feet, or both can occur. Within 2 to 3 weeks after the onset of fever, desquamation of the fingers or toes usually starts in periungual regions and may spread to the palms or soles. Nail changes, such as, deep transverse grooves across nails (Beau's lines) may occur at 1 to 2 months after fever onset. On the other hand, erythematous rashes appear within 5 days of fever onset, and consist mostly of diffuse maculopapular eruptions, urticarial exanthems, scarlatiniform rashes, erythroderma, or occasionally erythema-multiforme-like rashes, which usually involve extensive areas of the trunk and extremities. Furthermore, these rashes are known to be accentuated in perineal regions, where early desquamation may also occur6.
In the present case, desquamative skin lesions had developed on the palms, soles, and perineal regions at the time of diagnosis of KD (on admission). About one week later, depigmented lesions occurred on previous desquamative areas during the KD convalescent phase. Vitiligo is known to be associated with many diseases. However, to the best of our knowledge, no previous report has associated vitiligo and KD. The nature of this association is unclear, in particular, it is not known whether there is a cause and effect relation or whether the two were coincidental in our patient. Nevertheless, the etiologies of both diseases are believed to have an immunologic basis. Thus, the co-occurrence of the two in our patient suggests that vitiligo and KD are immunologically associated.
Figures and Tables
 | Fig. 1Characteristic erythematous, desquamative maculopatches on the fingertips (A) and perianal skin (B) on Feb. 10, 2007, when he was admitted to our hospital. |
References
2. Kemp EH, Gavalas NG, Gawkrodger DJ, Weetman AP. Autoantibody responses to melanocytes in the depigmenting skin disease vitiligo. Autoimmun Rev. 2007. 6:138–142.

3. Passeron T, Ortonne JP. Physiopathology and genetics of vitiligo. J Autoimmun. 2005. 25:Suppl. 63–68.

4. Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003. 16:90–100.

5. Harning R, Cui J, Bystryn JC. Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol. 1991. 97:1078–1080.

6. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004. 114:1708–1733.

7. Zvulunov A, Greenberg D, Cagnano E, Einhorn M. Development of psoriatic lesions during acute and convalescent phases of Kawasaki disease. J Paediatr Child Health. 2003. 39:229–231.

8. Schwartz RA, Janniger CK. Vitiligo. Cutis. 1997. 60:239–244.
11. Frieden IJ, Resnick SD. Kawasaki disease. Pediatr Clin North Am. 1991. 38:881–882.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download