Abstract
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare benign vasoproliferative disease of an unknown cause involving the skin or subcutaneous tissue of the head and neck, and particularly around the ear. It predominantly affects Caucasian adults during the third and fourth decades and it very rarely occurs in children. We experienced a case of ALHE in a 2-year-old Korean boy who had a firm, pruritic, skin-colored, subcutaneous nodule on his right upper arm. The histopathological findings were compatible with ALHE and they showed prominent vascular changes with epitheloid or histiocytoid endothelial cells surrounded by inflammatory cells, including a large proportion of eosinophils. This unusual distribution of the lesion and the young age of the patient may be associated with vaccination.
Angiolymphoid hyperplasia with eosinophilia was first described by Wells and Whimster in 19691. It may be solitary or multiple, and it usually presents as superficial, light pink to red-brown papules and nodules that are frequently found in the dermis and superficial fascia of the head and neck, and particularly in the preauricular region2-4. It occurs predominantly in Caucasian women during the third and fourth decades and it is uncommon in children. Histologically, it is a benign vascular lesion that's characterized by the proliferation of atypical endothelial cells in combination with an eosinophilic infiltrate and lymphoid aggregates5.
Western authors regard Kimura's disease and angiolymphoid hyperplasia with eosinophilia as the same disease because they have similar features: head and neck lesions, lymphoid infiltration, vascular proliferation and tissue eosinophilia. However, it now widely accepted that these are two separate disease entities.
We report here on a case of angiolymphoid hyperplasia with eosinophilia in a 2-year-old Korean boy with an atypical lesion location on the upper right arm. The site of the lesion was around the site of a prior vaccination. This unusual distribution of the lesion and the young age of the patient were probably associated with vaccination.
A 2-year-old Korean boy presented with a solitary, skin-colored, hard, subcutaneous nodule that was located on his right upper arm. The lesion was detected by chance one week previously. He suffered from occasional pruritus.
On physical examination, the lesion in the right deltoid region was firm, non-tender, dome-shaped and it measured 20 mm in diameter, with normal overlying skin (Fig. 1). There was no regional lymphadenopathy and no other abnormality on examination. A wide excision biopsy was taken, and the sections of the biopsy specimen were stained with hematoxylin-eosin, which revealed prominent vascular proliferation, multiple lymphoid aggregates without germinal centers and polymorphous inflammatory cell infiltrates of the dermis and subcutaneous tissue. A diffuse inflammatory infiltrate with lymphocytes, plasma cells and abundant eosinophils surrounded the endothelial cell proliferations and vascular channels (Fig. 2A). The vascular walls were made up of prominent endothelial cells with an epitheloid or histiocytoid appearance, and these cells projected into the lumen (Fig. 2B). No mitoses or atypical cells were observed. Immunohistochemistry showed positive staining of the histiocytoid endothelial cells with factor VIII-related antigen (Fig. 3), the nodular and diffuse T-cell infiltrate stained for CD3 and the diffuse B-cell infiltrate stained for CD20 (Fig. 4). No recurrence has been reported after one year of follow-up.
Angiolymphoid hyperplasia with eosinophilia (ALHE) is a rare benign vascular inflammatory lesion with a prominent proliferation of atypical endothelial cells and an infiltrate that contains numerous eosinophils5. It was first described in 1969 by Wells and Whimster1, who thought that Kimura's disease and ALHE represented two ends of the same disease spectrum, i.e., an initial marked vessel proliferation and later a lymphocyte proliferation. However, Rosai et al6 recognized that Kimura's disease and ALHE differed in terms of their histopathological features and they suggested that they are distinct entities. Therefore, they are now regarded as two distinct diseases7,8.
Clinically, ALHE presents as single or multiple pink to reddish-brown papules or subcutaneous nodules that are usually located on the head and neck, and especially in the preauricular region, and ALHE generally occurs in young adults9. It has been described less frequently at other sites10,11 and in children12,13. Histopathologically, ALHE is characterized by the proliferation of blood vessels lined by plump epitheloid or histiocytoid endothelial cells that protrude into the lumen, and this occasionally results in a cobblestone appearance. The vascular proliferations are surrounded by a mixed inflammatory infiltrate that predominantly consists of eosinophils, but the infiltrate includes plasma cells and lymphocytes4,8. By contrast, Kimura's disease is characterized by multiple lymphoid follicles with germinal centers and eosinophilic microabscesses (Table 1)8.
In our case, the histology showed multiple lymphoid follicles without germinal centers in the dermis and subcutaneous tissue, there were atypical endothelial cells and positive staining for factor VIII-related antigen, which is all compatible with ALHE. The same as Helander et al14 reported, a diffuse T-cell and B-cell infiltration was observed in our case.
The pathogenesis of ALHE is unknown, with opinions varying between it being a benign vascular neoplasm to a reactive inflammatory lesion in the form of an atopic reaction to various agents or trauma. It has recently been considered a reactive inflammatory disease secondary to an immunological mechanism. This is supported by the immunoglobulin deposition within blood vessels, the increased serum cryoglobulin levels, the blood and tissue eosinophilia, the elevated serum IgE concentrations and the slight predominance in atopic patients15.
Our patient is of interest because of the unusual site and his young age. The development in a child, the atypical location on the right upper arm and the remarkable correlation between the injection site and the location of the lesion suggests an etiological role for vaccination. We hypothesize that a persistent inflammatory response secondary to damage to vessels, or a vaccination or immunological reaction or both are most likely involved in the development of ALHE in our patient. Although it is difficult to demonstrate a direct etiological role for vaccination in the development of ALHE, vaccination may be one of multiple factors that promote an inflammatory reaction and vascular proliferation that can result in the development of ALHE.
The treatment of choice for ALHE is complete surgical excision, although recurrences are common16. Therefore, in a child, ALHE must be included in the differential diagnosis of nodular lesions that occur at vaccination sites such as the arms and thighs.
Figures and Tables
 | Fig. 2(A) Several lymphoid aggregates without germinal centers showing lymphocyte and eosinophil infiltration (H&E, ×40). (B) Vascular proliferation with "epitheloid" or "histiocytoid" endothelial cells. There is a cobblestone appearance of enlarged endothelial cells that project into the vessel lumen and a perivascular cellular infiltration that mainly consists of eosinophils and lymphocytes in the dermis (H&E, ×100). |
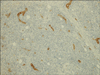 | Fig. 3Positive staining for factor VIII-related antigen (immunoperoxidase, ×100) on the walls of the proliferating vessels. |
References
1. Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969. 81:1–14.

2. Kennedy CTC. Champion RH, Burton JL, Burns DA, Breathnach SM, editors. The external ear. Rook/Wilkinson/Ebling textbook of dermatology. 1998. 6th ed. Malden: Blackwell Science;3027–3028.

3. Iguchi Y, Inoue T, Shimono M, Yamamura T, Shigematsu T, Takahashi S. Kimura's disease and its relation to angiolymphoid hyperplasia with eosinophilia: report of three cases and review of the literature. J Oral Pathol. 1986. 15:132–137.

4. Googe PB, Harris NL, Mihm MC Jr. Kimura's disease and angiolymphoid hyperplasia with eosinophilia: two distinct histopathological entities. J Cutan Pathol. 1987. 14:263–271.

5. Mehregan AH, Shapiro L. Angiolymphoid hyperplasia with eosinophilia. Arch Dermatol. 1971. 103:50–57.

6. Rosai J, Gold J, Landy R. The histiocytoid hemangiomas. A unifying concept embracing several previously described entities of skin, soft tissue, large vessels, bone, and heart. Hum Pathol. 1979. 10:707–730.
7. Urabe A, Tsuneyoshi M, Enjoji M. Epithelioid hemangioma versus Kimura's disease. A comparative clinicopathologic study. Am J Surg Pathol. 1987. 11:758–766.
8. Chun SI, Ji HG. Kimura's disease and angiolymphoid hyperplasia with eosinophilia: clinical and histopathologic differences. J Am Acad Dermatol. 1992. 27:954–958.

9. Henry PG, Burnett JW. Angiolymphoid hyperplasia with eosinophilia. Arch Dermatol. 1978. 114:1168–1172.

10. Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. A clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985. 12:781–796.
11. Arnold M, Geilen CC, Coupland SE, Krengel S, Dippel E, Sproder J, et al. Unilateral angiolymphoid hyperplasia with eosinophilia involving the left arm and hand. J Cutan Pathol. 1999. 26:436–440.

12. Akosa AB, Ali MH, Khoo CT, Evans DM. Angiolymphoid hyperplasia with eosinophilia associated with tetanus toxoid vaccination. Histopathology. 1990. 16:589–593.

13. Hallam LA, Mackinlay GA, Wright AM. Angiolymphoid hyperplasia with eosinophilia: possible aetiological role for immunisation. J Clin Pathol. 1989. 42:944–949.

14. Helander SD, Peters MS, Kuo TT, Su WP. Kimura's disease and angiolymphoid hyperplasia with eosinophilia: new observations from immunohistochemical studies of lymphocyte markers, endothelial antigens, and granulocyte proteins. J Cutan Pathol. 1995. 22:319–326.

15. Haas AF, La Perriere R, King E. Angiolymphoid hyperplasia with eosinophilia of the hand. A case report. J Dermatol Surg Oncol. 1991. 17:731–734.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download