Abstract
Congenital leukemia is a rare disease that develops from birth to 6 weeks of life. Leukemia cutis involves cutaneous infiltration by leukemic cells and is an unusual manifestation of leukemia, and has been documented in 25~30% of patients with congenital leukemia. The authors report a case of congenital leukemia cutis. A newborn male presented with widespread firm dusky red papules and nodules on almost his entire body surface. Skin biopsy specimens confirmed the presence of leukemic infiltrations, and bone marrow cytology was consistent with acute myeloid leukemia of the FAB M5 type.
Congenital leukemia is a rare condition that is diagnosed from birth to 6 weeks of life1. It occurs at a rate of 1 per 5 million births and represents less than 1% of all childhood leukemia2-4. Leukemia cutis is typified by cutaneous infiltrates of leukemic cells and is an uncommon manifestation of leukemia. It has been documented in 25~30% of patients with congenital leukemia5,6. In childhood and adult leukemia, leukemia cutis typically develops late during the disease course and is strongly associated with the presence of extramedullary disease at other sites. We report a case of congenital leukemia cutis which was confirmed as acute myeloid leukemia (AML) of the French-American-British (FAB) M5 type.
A male infant with a birth weight of 3,360 g and a height at birth of 50 cm was born on gestation week 38. His mother was a healthy 32-year-old; blood type AB positive, hepatitis B surface antigen negative, hepatitis B surface antibody positive, venereal disease research laboratory (VDRL) test negative, and human immunodeficiency virus (HIV) antibody negative. Furthermore, there was no known history of maternal drug use or exposure to known teratogens. She delivered a normal baby by cesarean section 3 years ago and had undergone an abortion 4 years ago. Family history findings were non-specific. Cesarean section had been performed due to fetal distress. A physical examination of this newborn revealed unstable respiration with mild tachypnea, and a skin examination revealed widespread dusky red papules and nodules (0.5~2 cm sized in diameter) with purpuric macules and ecchymoses predominantly on his scalp, face, neck, upper chest and both arms (Fig. 1).
His hemoglobin was 13.9 g/dl, platelet count 133×103/UL, hematocrit 44.5%, and white blood cells (WBC) count 173×103/UL, and a differential count revealed neutrophils 58.8%, lymphocytes 5.3%, monocytes 26.1%, eosinophils 0.5%, and basophils 3.7%. Blood urea nitrogen (BUN) was 5.9 mg/dl, creatinine 0.7 mg/dl, aspartate aminotransferase (AST) 82 IU/L, alanine aminotransferase (ALT) 19 IU/L, lactate dehydrogenase (LD) 2,354 IU/L, alkaline phosphates (ALP) 412 IU/L, gamma glutamyl transferase (GGT) 229 IU/L, total bilirubin 2.5 mg/dl, direct bilirubin 0.5 mg/dl. His blood group was A positive, his prothrombin time (PT) was 14.8 seconds and activated partial thromboplastin time (aPTT) 44.9 seconds. Serology studies for TORCH syndrome, which included serum antibody titers against toxoplasmosis, cytomegalovirus (CMV), rubella, and herpes simplex virus were negative. Chest and abdominal radiographs were normal.
Hematoxylin-eosin staining of a right forearm skin biopsy specimen demonstrated a dense, diffuse, atypical infiltrate, which replaced the entire dermis and extended into subcutaneous fat but spared the epidermis. The cells were characterized by a large pleomorphic round to oval nucleus with one or more discrete nuclei and abundant pale cytoplasm (Fig. 2). These cells stained positively for leukocyte common antigen (LCA), but were negative for CD3, CD20, CD34, CD45 and CD68.
A peripheral blood smear revealed a normochromic macrocytic RBC morphology and WBCs showed marked leukocytosis (32% immature cells and 40% monocytes) (Fig. 3A). A bone marrow examination showed that the marrow space was packed with leukemic blasts and immature monocytes. Blasts accounted for about 58% and normal hematopoietic precursors were markedly decreased. Leukemic cells frequently contained azurophilic granules (Fig. 3B-D). Peroxidase staining was weakly positive, but nonspecific esterase (NSE) and neutrophil activating factor (NaF) were positive (Fig. 3E, F). A leukemic marker study revealed CD33, CD14, and HLA-DR positivity. The infant was diagnosed, according to the FAB system, as having congenital acute myelogenous leukemia (acute monocytic leukemia, AML-M5). Bone marrow cytogenetic analysis was performed on 21 cells. All cells were of the 46 XY karyotype, but 10 cells are normal karyotype and 11 cells are abnormal. Chromosomal abnormalities including a translocation between chromosomes 8 and 16 and an abnormality on chromosome 19 were detected using the MTX-synchronized high-resolution banding technique and the thymidine-treated unsynchronized culture technique. Fluorescence in situ hybridization (FISH) would have been required to evaluate the abnormality in chromosome 19, but this was unavailable.
The infant was treated conservatively without chemotherapy or bone marrow transplantation, due to parental refusal. He died of respiratory distress with pulmonary infiltration at 28 days after birth.
The term congenital leukemia was originally applied to cases of leukemia that developed at birth, whereas neonatal and infantile leukemia were used to describe leukemia developing within the first 4 weeks of life or from 4 weeks to 1 year of life, respectively. However, commonly congenital leukemia is diagnosed from birth to 6 weeks of life. Patients with congenital leukemia fulfill the following diagnostic criteria1: First, a finding of immature cell proliferation in myelomonocytic, lymphoid, or erythroid series; and second, infiltration of these cells into extrahematopoietic tissue. Third, the absence of any other disease that might cause leukemoid reactions mimicking congenital leukemia, such as, TORCH syndrome, hemolytic disease of the newborn (ABO or Rh incompatibility), hereditary spherocytosis, twin-twin transfusion, other neoplastic infiltrates (metastatic neuroblastoma, rhabdomyosarcoma, Langerhans cell histiocytosis) and the absence of Down's syndrome or cytogenetic abnormalities of chromosome 21 with a transient abnormal myeloproliferative disorder. The majority of cases of congenital leukemia are of the myelogenous type, and the notable more common varieties of congenital leukemia are the myelomonocytic (M4) or monocytic (M5) FAB subtypes, and rarely the lymphoblastic subtypes7,8.
Congenital leukemia is often associated with pallor, lethargy, hepatosplenomegaly, leukemia cutis, anemia, and leukocytosis. Leukemia cutis occurs in approximately 10% of adults with AML and in 1% of those with acute lymphoblastic leukemia (ALL)9. On the other hand, it has been documented to occur in 25~30% of patients with congenital leukemia5,6. According to the FAB system, most cases of congenital leukemia cutis are of the M5 subtypes8. Congenital leukemia cutis has been reported in 11 cases in Korea10-12, and of these, 6 were diagnosed by bone marrow biopsy. According to the FAB system, one of the six was M3, another was M4, and the others were M5. Our case was also M5, and thus, we believe that M5 is likely to be the most common type in Korea. The specific clinical morphology of leukemia cutis in congenital leukemia has been reported to be variable. However, the predominant morphology is of red to purple colored dermal nodules, though some reports mention additional tumors, papules, and macules. Lesions are typically multiple with a generalized distributed pattern. Involvements of the oral and ocular mucosa are quite rare.
The causes of congenital leukemia are unknown, but it has been associated with maternal exposure to radiation, maternal dietary exposure to bioflavonoid, maternal use of tobacco and illicit drugs, and inherited conditions, such as, Down's syndrome, neurofibromatosis, Bloom's syndrome, and Fanconi's anemia13. Chromosomal instability is a hallmark of congenital leukemia, and the most common karyotypic abnormality involves the myeloid-lineage leukemia gene at the 11q23 translocation breakpoint7,14. However, this translocation can involve several different chromosomal partners, such as, 4, 9 and 191. Evaluations of infants suspected of having leukemia cutis should include skin biopsy, serology, peripheral smear, bone marrow aspirate, and cytogenetics1, and the differential diagnosis should include congenital infections (rubella, Coxsackie's virus, CMV, and toxoplasmosis), hemolytic disease, transient myeloproliferative disorder and other infiltrative cutaneous processes, such as, metastatic neuroblastoma, malignant histiocytosis, and Langerhans cell histiocytosis15.
Histopathologic analysis of leukemia cutis lesions may reveal different patterns of infiltration16. Most commonly, a dense diffuse dermal infiltrate of pleomorphic leukemic cells is observed in a linearly array between collagen bundles in the reticular dermis. Some, band-like or compact nodular infiltrates may also be seen. These infiltrates show extension into the subcutis in most cases, but rarely infiltrate the epidermis. Malignant myeloid cells are the dominant cell type of acute myeloid leukemia cutis and tend to appear monotonous and homogeneous cytologically. Nuclei are round to oval, chromatin is likely to be evenly dispersed, and nucleoli are multiple but potentially inconspicuous. Despite these suggestive histological characteristics, histochemistry and immunohistochemistry are generally required for confirmation, in addition to examinations of peripheral blood and bone marrow.
Congenital leukemia has a poor prognosis with an overall survival rate of only 20% at 2 years of age6,7. However, unlike leukemia cutis in adults, which confers a poor prognosis for 90% patients with extramedullary involvements, cutaneous involvement in congenital leukemia does not uniformly portend a poor prognosis17, and a few reported patients have undergone spontaneous remissions of congenital AML without chemotherapeutic intervention1,18,19. On the other hand, aggressive chemotherapy and bone marrow transplantation can result in high morbidity and mortality rates1.
We report a male neonate born with congenital acute myeloid leukemia who presented with leukemia cutis. This case displayed highly aggressive skin infiltration with widespread dusky red papules to nodules from birth and a very poor prognosis.
Figures and Tables
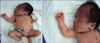 | Fig. 1(A) Multiple scattered dusky red papules and nodules were distributed over the entire body. (B) Diffuse purpuric macules and ecchymoses predominated on scalp, face, and neck. |
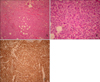 | Fig. 2(A) Biopsy of a skin nodule on the right arm showing dense, diffuse, and atypical infiltration of leukemic cells into dermis and subcutaneous fat (Hematoxylin-eosin, ×100). (B) The majority of infiltrating cells were large pleomorphic cells with a round to oval nucleus with one or more discrete nuclei and abundant pale cytoplasm (Hematoxylin-eosin, ×400). (C) Leukemic cells stained positively for leukocyte common antigen (Immunohistochemical staining, ×100). |
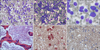 | Fig. 3(A) Peripheral blood smear showing markedly elevated monoblast and promonocyte numbers. (B), (C) Bone marrow aspiration showing promonocytes and monocytes containing azurophilic granules. (D) Bone marrow biopsy finding showing that the marrow space was packed with leukemic blasts and immature monocytes. (E), (F) The peripheral blood smear was positive for nonspecific esterase (NSE) and neutrophil activating factor (NaF). |
References
1. Landers MC, Malempati S, Tilford D, Gatter K, White C, Schroeder TL. Spontaneous regression of aleukemia congenital leukemia cutis. Pediatr Dermatol. 2005. 22:26–30.

2. Zhang IH, Zane LT, Braun BS, Maize J Jr, Zoger S, Loh ML. Congenital leukemia cutis with subsequent development of leukemia. J Am Acad Dermatol. 2006. 54:S22–S27.

4. Lampkin BC. The newborn infant with leukemia. J Pediatr. 1997. 131:176–177.
5. Torrelo A, Madero L, Mediero IG, Bano A, Zambrano A. Aleukemic congenital leukemia cutis. Pediatr Dermatol. 2004. 21:458–461.

6. Resnik KS, Brod BB. Leukemia cutis in congenital leukemia. Analysis and review of the world literature with report of an additional case. Arch Dermatol. 1993. 129:1301–1306.

7. Bresters D, Reus AC, Veerman AJ, van Wering ER, van der Does-van den Berg A, Kaspers GJ. Congenital leukaemia: the Dutch experience and review of the literature. Br J Haematol. 2002. 117:513–524.

8. Crist WM, Pui CH. Nelson WE, Behrman RE, Kliegman RM, Arvin AM, editors. The leukemia. Nelson textbook of pediatrics. 1996. 15th ed. Philadelphia: W.B. Saunders;1452–1457.
9. de Lacerda JF, do Carmo JA, Guerra ML, de Almeida LS, Fernandes A, de Lacerda JM. Leukemia cutis in acute lymphoblastic leukemia. J Am Acad Dermatol. 1994. 30:1041–1043.

10. Hur J, Kin YS, Yu HJ, Kim JS. A case of congenital leukemia cutis. Ann Dermatol. 2008. 20:74–76.

11. Chang SE, Koh KJ, Choi JH, Sung KJ, Moon KC, Koh JK. Congenital leukemia with a leukemic infiltration of skin. Korean J Dermatol. 2000. 38:702–704.
12. Ro YS, Moon DG, Lee CW, Han HG, Lee H, Choi JK. A case of congenital leukemia cutis. Korean J Dermatol. 2000. 38:1089–1093.
13. Bayoumy M, Wynn T, Jamil A, Kahwash S, Klopfenstein K, Ruymann F. Prenatal presentation supports the in utero development of congenital leukemia: a case report. J Pediatr Hematol Oncol. 2003. 25:148–152.

14. Cimino G, Lo Coco F, Biondi A, Elia L, Luciano A, Croce CM, et al. ALL-1 gene at chromosome 11q23 is consistently altered in acute leukemia of early infancy. Blood. 1993. 82:544–546.

15. Loh ML, Matthay KK. Taeusch HW, Ballard RA, Gleason CA, editors. Congenital malignant disorders. Avery's diseases of the newborn. 2005. 8th ed. Philadelphia: Elsevier Saunders;1450.

16. Longacre TA, Smoller BR. Leukemia cutis. Analysis of 50 biopsy-proven cases with an emphasis on occurrence in myelodysplastic syndromes. Am J Clin Pathol. 1993. 100:276–284.

17. Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994. 13:223–230.
18. Grundy RG, Martinez A, Kempski H, Malone M, Atherton D. Spontaneous remission of congenital leukemia: a case for conservative treatment. J Pediatr Hematol Oncol. 2000. 22:252–255.

19. Lampkin BC, Peipon JJ, Price JK, Bove KE, Srivastava AK, Jones MM. Spontaneous remission of presumed congenital acute nonlymphoblastic leukemia (ANLL) in a karyotypically normal neonate. Am J Pediatr Hematol Oncol. 1985. 7:346–351.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download