INTRODUCTION
CASE REPORT
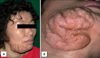
A 48-year-old female presented with a pruritic skin-colored soft tumor on her right lower cheek that had appeared as small papules 20 years earlier and had been growing gradually. The skin lesion was a skin-colored, cerebriform mass, sized 10×8×5 cm, on the right mandibular area (Fig. 1A). Its smooth surface contained scattered, dilated hair follicle-like pores with a waxy discharge (Fig. 1B).
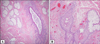
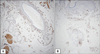
Histological examination showed numerous sebaceous lobules radiating from cystic follicular structures with mesenchymal changes. Numerous mature sebaceous lobules surrounded the cystic structures. The cystic structures were lined with stratified squamous epithelium and showed well-developed, dilated infundibular portions of hair follicles with infundibular keratinization. There were some increases of muscle components near the sebaceous lobules (Fig. 2A), but no secondary hair follicles. The folliculo-cystic structures were wrapped in densely laminated collagen bundles with various vascular proliferation (Fig. 2B). In immunohistochemical staining, muscle components were immunoreactive to desmin and smooth muscle actin (Fig. 3A). Increased vascular structures showed reactivity to CD31 (Fig. 3B), but tumor stromal cells were negative for S-100 protein and neurofilament.
The size of the lesion decreased slightly after treatment with isotretinoin (30 mg/d) for 3 months, but returned to its original size after cessation of drug. We recommended that the patient undergo excision and skin grafting but she did not want further treatment.
DISCUSSION
FSCH is a distinct cutaneous hamartoma formed from epithelial and stromal elements. Among the cases of FSCH in the literature1-20, only a few cases of giant FSCH have been reported (Table 1)3-6. The clinical features of our case were similar to those of the reported giant FSCH cases, except for cerebriform surface and chin involvement. FSCH is slightly more common in females. The sites of predilection are the nose, cheeks, forehead, and scalp. In most cases, the lesion does not exceed 3 cm in diameter7. The four cases in the literature described as "giant" ranged from 5 cm to 23 cm in diameter. The lesions in giant FSCH cases occurred in various sites, such as genital areas, upper extremities, and upper back.
FSCH has distinct, unique, classical histopathologic features. Kimura et al1 established the histological criteria for diagnosis of FSCH as: 1. an infundibular cystic structure attached to sebaceous lobules via sebaceous ducts; 2. laminated fibroplasias around the entire fibroepithelial units; 3. mesenchymal changes around fibroepithelial units; 4. clefts between fibroepithelial units and surrounding altered stroma; 5. confinement of the process primarily to the dermis. Several variations in FSCH reflect the predominance of either epithelial or non-epithelial components. Fogt and Tahan8 reported large expanses of tufted aggregates, separated or enveloped within the epithelial units. Donati and Balus9 presented a case with neural proliferation, while Aloi et al10 described copious mucin deposits in a fibrillary stroma. Our case involved several mesenchymal changes, such as laminated collagen, vascular proliferation, and slightly increased muscle components.
Because of its peculiar histopathologic features, diagnosis of FSCH is not difficult. But, in some cases, FSCH must be differentiated from sebaceous trichofolliculoma. Sebaceous trichofolliculoma is associated with pilosebaceous hyperplasia and overgrowth5. It presents as a central cystic cavity, usually containing a hair shaft, with sebaceous lobules and secondary hair follicles. However, sebaceous trichofolliculoma has minimal surrounding stromal proliferation. In FSCH, there are no secondary hair follicles and hair shaft in the cystic cavity. Mesenchymal change in FSCH is another point of differential diagnosis.
Giant FSCH is less common than ordinary FSCH and this particular case occurred on the face unlike other giant FSCH cases. We need to gather more cases to determine how FSCH and giant FSCH differ from each other in clinical features and prognosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download