Abstract
Background
No studies have yet been undertaken to determine the effect of aloe gel on the clinical signs and biochemical changes of aging skin.
Objective
We wanted to determine whether dietary aloe vera gel has anti-aging properties on the skin.
Methods
Thirty healthy female subjects over the age of 45 were recruited and they received 2 different doses (low-dose: 1,200 mg/d, high-dose: 3,600 mg/d) of aloe vera gel supplementation for 90 days. Their baseline status was used as a control. At baseline and at completion of the study, facial wrinkles were measured using a skin replica, and facial elasticity was measured by an in vivo suction skin elasticity meter. Skin samples were taken before and after aloe intake to compare the type I procollagen and matrix metalloproteinase 1 (MMP-1) mRNA levels by performing real-time RT-PCR.
Results
After aloe gel intake, the facial wrinkles improved significantly (p<0.05) in both groups, and facial elasticity improved in the lower-dose group. In the photoprotected skin, the type I procollagen mRNA levels were increased in both groups, albeit without significance; the MMP-1 mRNA levels were significantly decreased in the higher-dose group. Type I procollagen immunostaining was substantially increased throughout the dermis in both groups.
Conclusion
Aloe gel significantly improves wrinkles and elasticity in photoaged human skin, with an increase in collagen production in the photoprotected skin and a decrease in the collagen-degrading MMP-1 gene expression. However, no dose-response relationship was found between the low-dose and high-dose groups.
Skin aging is attributed to intrinsic (chronological) aging and photoaging (extrinsic aging). Photoaging and intrinsic aging are induced by damage to human skin by repeated exposure to ultraviolet (UV) irradiation and the damage due to the passage of time, respectively. An alteration in collagen, which is the major structural component of skin, has been considered to be a cause of skin aging in naturally aged and photoaged skin. With increasing age, there is a sustained reduction of collagen and an elevated secretion of matrix-degrading enzymes called matrix metalloproteinases (MMP) in old skin as compared with young skin1. UV causes photoaging by generating reactive oxygen species, and this subsequently triggers a cascade of signaling mechanisms and this eventually causes a decrease of collagen, and an increase of MMP, inflammation, epidermal DNA damage and apoptosis. During this process, activator protein 1 (AP-1) is activated by UV irradiation and so AP-1-driven MMPs such as MMP-1 and MMP-9 are induced. The UV-induced MMPs can degrade collagen, which results in a collagen deficiency in photodamaged skin and eventually skin wrinkling2.
The discovery or development of a novel agent that can delay the appearance of wrinkles and other features of cutaneous aging has been the quest of the pharmaceutical and cosmeceutical industries. Aloe barbadensis is commercially known as aloe vera, and it is a substance for which claims have been made about its anti-inflammatory, healing, moisturizing, antibacterial, antifungal and antiviral properties3-5. The gel is obtained from the pulp of aloe vera, a tropical cactus that belongs to the lily family, and aloe has been used as folk remedy since Roman times and it is now a familiar ingredient in a wide range of healthcare and cosmetic products. The gel comprises the inner, colorless part of the aloe vera leaf, and the exudate from the outer layers is also used for therapeutic purposes. Different gel constituents, including salicylates4, magnesium lactate3, bradykinin or thromboxane6, and polysaccharides7,8 have been presented as the reasons for aloe gel's efficacy. However, the biochemical basis for its action or influence on tissue repair is just beginning to be understood.
The reports concerned with the wound-healing effect of aloe in experimental animals or humans have been contradictory. In one such study, the incision wounds in rats were rapidly healed by aloe gel and the effect was attributed to more rapid maturation of collagen5. Other researchers have suggested that aloe increases the oxygen access of tissues as a result of an increased blood supply9 and others have suggested that aloe stimulates fibroblast activity and collagen proliferation10. Mannose-6-phosphate was identified as a cause of significant wound healing by aloe11, and the healing by aloe was found to be accompanied by higher levels of hyaluronic acid and dermatan sulfate, which were suggested to stimulate collagen synthesis and fibroblast activity12. On the other hand, controlled clinical trials in humans demonstrated no benefit when aloe vera was incorporated into topical therapy13, and one study demonstrated delayed wound healing when aloe vera was incorporated14. Many of the inconsistent clinical results obtained for the therapeutic efficacy of aloe gel might have been caused by the history of the sample after removal from the leaf, or even the growing conditions of the plant15. No studies have yet been undertaken to determine the effect of aloe gel on the clinical signs and biochemical changes of cutaneous aging and photoaging.
In this study, we investigated whether dietary aloe vera gel supplementation affects facial wrinkles, elasticity and the mRNA levels of type I procollagen and MMP-1 in human skin in vivo, and we used a skin surface analyzing system and biochemical methods to determine this.
A total of 30 healthy female subjects over the age of 45 and who passed a screening exam were randomized to receive a low dose or a high dose of aloe. The exclusion criteria included topical corticosteroid or retinoid use 2 weeks prior to study entry and use of systemic steroid, vitamins or phototherapy 1 month prior to the study. The subjects were not allowed to use anti-wrinkle/whitening cosmetics, topical retinoids or chemical peels, or to take any other functional food or vitamins during the study. They were allowed to use sunscreen lotion with a Sun Protection Factor of 30 or higher.
The aloe vera gel liquid we used (manufacturer: Univera Company, Seoul, Korea) is obtained by dissolving concentrated aloe vera gel powder in distilled water with flavors. Two different concentrations of liquid were made from the aloe vera gel powder (lower-dose: 1%, higher-dose: 3%). The lower-dose group received 120 ml of 1% aloe vera liquid, which is equivalent to 1,200 mg of aloe vera gel/day; the higher-dose group received 120 ml of 3% aloe vera liquid, which translates to 3,600 mg of aloe vera gel/day.
A complete blood count (CBC), liver function tests (LFT) and urinalyses were conducted at baseline and at 90 days after beginning the study. The subjects were instructed to report any cutaneous or systemic adverse events after the initiation of the study. For the subjects who agreed to biopsies, skin samples were taken from the buttock skin before and after aloe intake. The specimens for RT-PCR analysis were snap-frozen in liquid nitrogen, and the specimens for immunohistochemical staining were oriented immediately in a cryomatrix (Shandon, Pittsburgh, PA, USA) and then this was stored at -70℃.
This study was conducted according to the principles of the Declaration of Helsinki. This study was approved by the Institutional Review Board at Seoul National University Hospital, and all the subjects gave their written informed consent.
At baseline and after 90 days, facial wrinkles were measured in the crow's feet area using a skin replica and a Visiometer SV 600 (Courage+Khazaka Electronic, Köln, Germany). The Visiometer is a computerized instrument that makes a skin microrelief map from the replica using a light transmission method. It has 5 roughness parameters: depth of roughness (R1), mean depth of roughness (R2), maximum roughness (R3), depth of smoothness (R4) and arithmetic average roughness (R5).
Facial elasticity was measured with a non-invasive, in vivo suction skin elasticity meter Cutometer MPA 580 (Courage +Khazaka Electronic, Köln, Germany). The Cutometer takes measurements based on the principle of suction elongation, using an optical measuring unit. Of the measured and calculated R-parameters taken with the Cutometer, certain ratios of the parameters are biologically meaningful and these do not depend on skin thickness, and so they can be compared between different skin sites and subjects. In particular, R2 (gross elasticity), R5 (net elasticity) and R7 (elasticity/complete curve) are known to be indicators of skin elasticity, and the closer each value is to 1, the more elastic the skin is.
All the measurements were performed in a controlled environment room, with a constant room temperature between 20 and 25℃ and the humidity was between 45 and 55%, at the Clinical Research Institute, Seoul National University Hospital.
The total RNA was extracted from tissues using TRIZOL reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), and 1µg of the total RNA was converted to cDNA using a First Strand cDNA Synthesis Kit (Roche Applied Science, Indianapolis, IN, USA). Quantitation of the procollagen α1 (I), the MMP-1 cDNA and the endogeneous reference GAPDH cDNA was performed using a fluorescence detection method (ABI PRISM 7000 Sequence Detection System, Perkin Elmer Applied Biosystems, Foster City, CA, USA). The sequence-specific PCR primer sets and a TaqMan MGB probe (FAM™ dye-labeled) were purchased from Applied Biosystems. The cycling conditions were 50℃ for 2 min, 95℃ for 10 min, followed by 40 cycles at 95℃ for 15 sec and 60℃ for 1 min. To quantify the relative changes in the gene expression between each sample, we used the comparative CT method, as was previously described16. In the comparative CT method, the ΔCT mean value obtained in the control sample is 0 and the fold difference is 1.
The samples were sectioned 4µm thick and fixed in acetone (5 min at -20℃). The sections were blocked with blocking solution (85-9043, Zymed, San Francisco, CA, USA) for 30 min and then they were incubated in a humidified chamber at 4℃ for 18 hours with monoclonal anti-human PIP antibody, which detects procollagen type I C-terminal peptide (M011, 1:1,000, Takara, Shiga, Japan). After washing in PBS, the sections were incubated with biotinylated secondary antibody (85-9043, Zymed) for 15 min. The sections were incubated with streptavidin (85-9043, Zymed) for 15 min, and the color reaction was performed with AEC (3-amino-9-ethylcarbazole; 00-2007, Zymed) developing solution for 5 to 10 min. The cell nuclei were then counterstained with Mayer's hematoxylin (S3309, Dako), and the samples were mounted using Faramount Aqueous Mounting Medium (S3025, Dako, USA).
The significance of differences between the higher and lower dose groups was analyzed by using the Mann-Whitney U-test for comparison of the baseline values between the two groups; Wilcoxon's signed rank test was used for comparison of the before- and after-aloe vera gel intake values in each group. For all the tests, a p value < 0.05 was considered significant.
All 30 subjects completed the trial without any adverse events. The ages of the 30 subjects ranged from 49 to 74 years (average age: 56.2 yrs), and their body weights ranged from 47 to 75 kg (average weight: 58.8 kg). In the lower-dose group (n=15), the average age was 57.8±7.1 years and the average weight was 55.9±5.8 kg; in the higher-dose group (n=15), the average age was 54.5±6.3 years and the average weight was 61.8±8.8 kg. By the Mann-Whitney test, there was no significant difference in the ages and body weights between the 2 groups, and so the selection of subjects was deemed appropriate. No subjective adverse events were reported. The laboratory evaluations revealed no significant abnormalities in the CBC, the LFTs and the urinalysis.
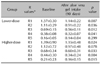
Comparison of the facial wrinkles before and after aloe gel intake, as measured by the skin replica and the Visiometer, is shown in Table 1. The Visiometer R values R1 thru R5 decrease as the wrinkles diminish, that is, the skin surface roughness decreases. Hence, the facial wrinkles in both groups were shown to have significantly decreased after 3 months of aloe vera supplementation, with the Visiometer R1 thru R4 values being decreased (p<0.05, Wilcoxon's signed rank test) in the lower-dose group, and the R1, R3-R5 values were significantly decreased in the higher-dose group.
The Cutometer was used to measure changes in skin elasticity. The closer the value is to 1, the more elastic the skin is. After 90 days of aloe vera supplementation, the R5 and R7 values were increased significantly in the lower-dose group (Table 2), indicating increased cutaneous elasticity. In the higher-dose group, the Cutometer R values all increased, but this was without statistical significance.
The type I procollagen and collagen-degrading MMP-1 gene expressions were compared by performing real-time RT-PCR. In the lower-dose group (n=6), the type I procollagen mRNA levels increased to 4.74±1.25 times the baseline level (p>0.05); in the higher-dose group (n=6), these levels increased 1.75±0.41 fold (p>0.05) (Fig. 1). The MMP-1 mRNA levels were nearly unchanged in the lower-dose group, with a 1.41±0.31 fold increase after aloe intake (n=6); however, in the higher-dose group, the MMP-1 mRNA transcripts were significantly (p<0.05, n=6) decreased to 0.51±0.13 times the baseline level (Fig. 2).
Type I procollagen immunostaining with anti-PIP antibody demonstrated substantially increased intracellular and extracellular procollagen expressions throughout the dermis after aloe vera gel intake in both treatment groups (Fig. 3).
This is the first clinical research to determine the effects of dietary aloe supplementation on facial wrinkles/elasticity and the type I procollagen and MMP-1 gene levels in aged human skin. In this study, we found that aloe vera gel supplementation clinically improved facial wrinkles and elasticity, while it increased type I procollagen and it decreased the MMP-1 gene expressions in the photoprotected skin.
In this study, the degree of clinical wrinkles was objectively measured by using a device (the Visiometer) that converts the skin surface roughness to numerical values. The facial wrinkles decreased in both the lower-dose and higher-dose groups; however, no dose-response relationship was seen in this study. This may be partially attributed to the small number of subjects in this study and a relatively short time period of aloe gel supplementation. The decrease in facial wrinkles as measured by the Visiometer reflects increased type I procollagen in both dosing groups. At the gene level, lower-dose aloe was shown to increase type I procollagen mRNA in the photo-protected buttock skin, albeit without statistical significance, whereas no change was seen in the MMP-1 mRNA levels. In the higher-dose group, while aloe gel had little effect on the type I procollagen mRNA levels, it caused a significant decrease in the MMP-1 mRNA levels. As a result, both dosing groups would have a net gain of procollagen that would correlate clinically with the improvement of wrinkles, as measured by the Visiometer. Type I procollagen immunostaining also demonstrated an upregulated protein expression in both groups.
The mechanism by which aloe exerts its anti-aging effects is unknown. The therapeutic action of aloe has been studied mostly for wound healing. When wounded diabetic rats were treated orally and topically with aloe gel, increased collagen formation was later demonstrated17, and the collagen formed had a higher degree of crosslinking, indicating enhanced levels of type III collagen18. According to the literature on the therapeutic effect of aloe, immunostimulation frequently appears as a contributory factor. Aloe gel extracts, when applied after UV exposure, were found to prevent suppression of local and systemic immunity to haptens and delayed type hypersensitivity responses to Candida albicans and alloantigens8,19. This was attributed to the presence of polysaccharides in the aloe vera gel. They have no significant anti-oxidant activity; the immune-protective action of aloe polysaccharides takes place at a step downstream from DNA damage and repair, possibly by modulating the DNA-damage-activated signal transduction pathways20. These compounds may act by novel mechanisms to block the signal transduction pathways and the production of immunosuppressive cytokines. An acetylated glucomannan in aloe was found to be the biologically active, dominant polysaccharide, so much so that it was named acemannan5. Acemannan from aloe was shown to increase collagen biosynthesis, and perhaps this occurred through macrophage stimulation21. In addition, active glycoproteins have been demonstrated in aloe gel and they may well play some part in aloe's therapeutic activity, either immunologically as lectins or as proteases such as anti-bradykinins. Superoxide dismutase activities have also been reported from Aloe vera gel22.
There are 3 cases of oral aloe vera-induced hepatitis in the medical literature23-25. In this study, no toxicity was observed in association with oral aloe intake. The doses used in the study were determined arbitrarily based on the daily recommended dosage of aloe by the manufacturer of the study material. From the two doses used in this study, no dose-response relationship could be demonstrated clinically (wrinkles and the elasticity measures), biochemically (procollagen and the MMP-1 gene levels) or microscopically (procollagen staining). Our results indicate no added advantage of high-dose aloe vera gel ingestion for cutaneous anti-aging purposes. The limitations of the study include the lack of a control group. In addition, daily sunblock use may have added to the protective effects of aloe; however, sunblock alone does not actively increase procollagen production or reduce the MMP-1 gene expression. It only renders skin less susceptible to further photodamage that would occur with the passage of time. Therefore, the role of sunblock as an active anti-aging substance could be excluded. For determining the optimal effective daily dosage of aloe, a placebo-controlled study with a larger number of subjects and a longer study period is warranted.
Since aloe significantly decreased wrinkles and it increased elasticity in photoaged human skin in vivo with an increase of the net procollagen, oral aloe gel supplementation may be a novel anti-aging strategy that prevents and repairs cutaneous photoaging. A future challenge will be to determine the mechanism of action of aloe gel in preventing cutaneous aging.
Figures and Tables
 | Fig. 1The type I procollagen mRNA levels measured by real-time RT-PCR before and after aloe vera gel intake in the lower-dose group (n=6) and the higher-dose group (n=6). Wilcoxon's signed rank test was used for statistical analysis. |
 | Fig. 2The collagen-degrading MMP-1 mRNA levels before and after aloe vera gel intake in the lower-dose group (n=6) and the higher-dose group (n=6). Statistical significance was tested by Wilcoxon's signed rank test. |
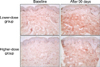 | Fig. 3Type I procollagen (Takara) immunostaining in the buttock skin before and after aloe vera intake (original magnification ×200). The results are representative of 6 biopsied subjects in each group. |
ACKNOWLEDGMENTS
The authors are indebted to Ae-Kyong Woo and Joo-Mi Shim for coordinating the study and procuring the cutaneous tissues. This research was supported by a grant from the Korean Food and Drug Administration.
References
1. Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000. 114:480–486.

2. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997. 337:1419–1428.

3. Shelton RM. Aloe vera. Its chemical and therapeutic properties. Int J Dermatol. 1991. 30:679–683.
6. Natow AJ. Aloe vera, fiction or fact. Cutis. 1986. 37:106. 108.
7. Strickland FM, Darvill A, Albersheim P, Eberhard S, Pauly M, Pelley RP. Inhibition of UV-induced immune suppression and interleukin-10 production by plant oligosaccharides and polysaccharides. Photochem Photobiol. 1999. 69:141–147.

8. Byeon SW, Pelley RP, Ullrich SE, Waller TA, Bucana CD, Strickland FM. Aloe barbadensis extracts reduce the production of interleukin-10 after exposure to ultraviolet radiation. J Invest Dermatol. 1998. 110:811–817.

9. Davis RH, Rosenthal KY, Cesario LR, Rouw GA. Processed Aloe vera administered topically inhibits inflammation. J Am Podiatr Med Assoc. 1989. 79:395–397.

10. Thompson JE. Topical use of aloe vera derived allantoin gel in otolaryngology. Ear Nose Throat J. 1991. 70:119.
11. Davis RH, Donato JJ, Hartman GM, Haas RC. Anti-inflammatory and wound healing activity of a growth substance in Aloe vera. J Am Podiatr Med Assoc. 1994. 84:77–81.

12. Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J Ethnopharmacol. 1998. 59:179–186.

13. Thomas DR, Goode PS, LaMaster K, Tennyson T. Acemannan hydrogel dressing versus saline dressing for pressure ulcers. A randomized, controlled trial. Adv Wound Care. 1998. 11:273–276.
14. Schmidt JM, Greenspoon JS. Aloe vera dermal wound gel is associated with a delay in wound healing. Obstet Gynecol. 1991. 78:115–117.
15. Yaron A. Characterization of Aloe vera gel before and after autodegradation, and stabilization of the natural fresh gel. Phytother Res. 1993. 7:S11–S13.
16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001. 25:402–408.

17. Chithra P, Sajithlal GB, Chandrakasan G. Influence of aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998. 59:195–201.

18. Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Mol Cell Biochem. 1998. 181:71–76.
19. Strickland FM, Pelley RP, Kripke ML. Prevention of ultraviolet radiation-induced suppression of contact and delayed hypersensitivity by Aloe barbadensis gel extract. J Invest Dermatol. 1994. 102:197–204.

20. Strickland FM. Immune regulation by polysaccharides: implications for skin cancer. J Photochem Photobiol B. 2001. 63:132–140.

21. Lindblad WJ, Thul J. Sustained increase in collagen biosynthesis in acemannan impregnated PVA implants in the rat [abstract]. Wound Repair Regen. 1994. 2:84.
22. Sabeh F, Wright T, Norton SJ. Isozymes of superoxide dismutase from Aloe vera. Enzyme Protein. 1996. 49:212–221.
23. Rabe C, Musch A, Schirmacher P, Kruis W, Hoffmann R. Acute hepatitis induced by an Aloe vera preparation: a case report. World J Gastroenterol. 2005. 11:303–304.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download