Abstract
Tuberculosis cutis orificialis (TCO) is a rare manifestation of cutaneous tuberculosis that is caused by auto-inoculation of mycobacteria in patients with advanced internal tuberculosis. TCO occurs in oral, perianal, or genital mucosa and adjacent skin. The tongue is the most frequently affected site, but the perianal area can also be affected. A 39-year-old male presented with a 4-month history of painful ulcers on the perianal area. The histopathologic findings revealed granulomatous infiltrates composed of epithelioid cells and Langhans-type giant cells in the dermis, and a few acid-fast bacilli noted on Ziehl-Neelsen staining. The polymerase chain reaction (PCR) was positive for Mycobacterium tuberculosis and the chest X-ray showed findings consistent with active pulmonary tuberculosis in both upper lung zones. The skin lesion showed complete resolution 2 months after the start of treatment with antituberculosis agents. We report a case of TCO with perianal involvement in a patient with underlying active pulmonary tuberculosis.
Tuberculosis cutis orificialis (TCO) is a rare manifestation of cutaneous tuberculosis which is characterized by painful ulcerative lesions affecting mucous membranes and adjacent skin of the orifices1. It occurs following auto-inoculation of mycobacteria in patients with advanced pulmonary and gastrointestinal tuberculosis. The tongue is the most frequently affected site1, but the perianal area can also be affected. To date, there have been 5 cases of TCO reported in the Korean dermatologic literature (Table 1)1-5. Herein we report a case of TCO involving perianal ulcerations in a 39-year-old male.
A 39-year-old Korean male presented with painful ulcers involving the perianal area for 4 months. One month ago he was treated with antibiotics and antiviral agents in a private clinic, but the skin lesions gradually worsened. His medical and family histories were unremarkable, except that he had an anal fistula repair 18 months ago. On the first visit, he looked acutely ill and cachectic, but did not have constitutional symptoms, such as fever, weight loss, night sweats, cough, or sputum. The skin examination revealed several sharply demarcated perianal ulcerations with erythematous indurated borders and purulent bases (Fig. 1A). Neither inguinal lymphadenopathy nor abnormalities on the digital rectal examination were noted. The laboratory tests, including a complete blood count, blood chemistry studies, and a urinalysis were normal, except the ESR (20 mm/hr) and CRP (1.87 mg/dl) were elevated. The results of a VDRL, HIV antibody by ELISA, and polymerase chain reaction (PCR) for herpes simplex virus were negative. A chest X-ray revealed multiple ill-defined nodular opacities in both upper lung zones (Fig. 1B). A skin biopsy obtained from the margin of the ulcer showed granulomatous infiltrates composed of epithelioid cells and Langhans-type giant cells (Fig. 2A) and caseous necrosis (Fig. 2B). A few acid-fast bacilli were noted in the granuloma by Ziehl-Neelsen staining (Fig. 2C). A culture from the ulcer exudates did not grow Mycobacterium tuberculosis, but a culture from the sputum grew M. tuberculosis. In addition, a PCR for M. tuberculosis obtained from the formalin-fixed, paraffin-embedded skin biopsy specimen was positive. Based on clinical, laboratory, and histopathologic findings, the patient was diagnosed with TCO with perianal involvement and underlying pulmonary tuberculosis. He was treated with antituberculosis agents, including isoniazid (300 mg/day), rifampicin (600 mg/day), pyrazinamide (1,500 mg/day), and ethambutol (1,200 mg/day). The perianal ulcers healed completely with scarring after 2 months of antituberculosis treatment. At that time, pyrazinamide was discontinued and the dose of ethambutol was reduced to 800 mg/day. He was treated with antituberculosis agents for a total of 9 months without a recurrence.
TCO is characterized by painful ulcers with indurated erythematous borders and necrotic bases in mucosal orifices of patients with advanced tuberculosis. The most common location of TCO is the oral mucosa, especially the tongue1. However, TCO can also occur in the perianal area, as in our case. The exact mechanism leading to TCO remains unknown. Sharma and Bhatia6 proposed four mechanisms for TCO: ingestion of bacilli in sputum from active pulmonary tuberculosis, hematogenous spread, lymphatic spread, and direct spread from adjacent organs. Of these putative mechanisms, ingestion of bacilli in sputum is the most common7. However, Akgun et al.8 presented a case of isolated perianal tuberculosis without pulmonary or gastrointestinal involvement.
Clinically, TCO usually presents with erythematous, edematous nodules or plaques. This is followed by painful central ulceration covered by necrotic, pseudomembranous materials with an irregular border. Most patients also have constitutional symptoms, such as fever, malaise, weight loss, and night sweats.
The histopathologic findings usually include ulceration surrounded by a non-specific inflammatory infiltrate, and extensive caseous necrosis. Granulomas composed of epithelioid cells and Langhans-type giant cells can also be seen in the dermis. Acid-fast bacilli can be detected using Ziehl-Neelsen staining. To obtain a more accurate diagnosis, cultures and PCR from skin biopsy specimens should be studied. PCR may allow a quicker and more precise diagnosis than cultures, but cultures should always be done to identify drug resistance9. In our patient, a PCR performed from the skin biopsy specimen led to an early diagnosis of TCO. The Mantoux test may be negative in patients with TCO due to a decrease in tuberculo-host defense10. Because most patients with TCO present with concomitant pulmonary tuberculosis, a chest X-ray should be performed. Ichihashi et al.7 reported that 9 patients concomitantly presented with pulmonary tuberculosis in 11 patients with TCO and perianal involvement (81.8%). Patients should also be evaluated with a barium enema, ultrasonography, computed tomographic scan, and colonoscopy to identify other gastrointestinal and peritoneal involvement6. These tests were not performed on the patient described herein due to an economic hardship.
Tuberculosis is a major opportunistic infection in HIV-infected patients and may worsen in the clinical course of HIV infection11. The mortality in patients with both HIV and tuberculosis is also higher than in those patients with HIV alone. Therefore, it is important to diagnose tuberculosis at an early stage in HIV-infected patients. Ghiya et al.12 reported three patients with HIV infection who presented with chronic, non-healing perianal ulcers originating from tuberculosis. If patients with HIV infection and perianal ulcers have antibiotic or antiherpetic treatment resistance, a chest x-ray, Mantoux test, acid-fast bacilli smear from the wound, cultures, and PCR should be performed12. The occurrence of tuberculosis is also related to other immunocompromised states. Lin et al.13 described a patient with angioimmunoblastic T-cell lymphoma who developed perianal tuberculosis during the neutropenic phase after chemotherapy.
Table 2 summarizes the differential diagnosis of TCO with perianal involvement14-16. Of these diagnoses, Crohn's disease is the most important entity within the differential diagnosis. These two diseases are similar in that they usually present with ulcers and/or ulcerated plaques and show granulomas on histopathologic examination8. However, TCO can be differentiated from Crohn's disease by Ziehl-Neelsen staining, culture, and PCR for tuberculosis.
TCO cannot resolve spontaneously and can lead to death due to miliary spread if not adequately treated. The initial treatment of TCO is a standard antituberculosis regimen, consisting of three or four agents. However, if the skin lesions do not respond to medication or are accompanied by obstruction and/or an abscess, a surgical approach is required14.
In our case, the patient had no history of tuberculosis and denied any constitutional symptoms associated with tuberculosis, except for cachexia. It was difficult to diagnose TCO until the histopathology results were obtained. In conclusion, we present a case of TCO with perianal involvement confirmed by PCR in a patient with underlying active pulmonary tuberculosis. TCO should be considered in the differential diagnosis of chronic painful perianal ulcers.
Figures and Tables
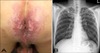 | Fig. 1(A) Several sharply demarcated ulcerations with erythematous indurated borders and purulent bases in the perianal area. (B) Chest x-ray film showing multiple ill-defined nodular opacities in both upper lung zones. |
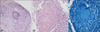 | Fig. 2Histopathologic examination of the margin of the ulcer revealed (A) granuloma composed of epithelioid cells and Langhans-type giant cells (H&E, ×200) and (B) caseous necrosis (H&E, ×200). (C) A few acid-fast bacilli were seen in the granuloma (Ziehl-Neelsen stain, ×400). |
References
1. Chung KY, Park SH, Lee SH. Tuberculosis cutis orificialis of the tongue. Korean J Dermatol. 1987. 25:802–805.
2. Kwon OJ, Han DS, Song JY. A case of tuberculosis cutis orificialis following pulmonary and intestinal tuberculosis. Korean J Dermatol. 1981. 19:427–432.
3. Baek SE, Kang WH, Lee KH. Tuberculosis cutis orificialis. Korean J Dermatol. 1985. 23:667–671.
4. Hong SM, Kim SN. A clinical and histopathologic study on skin tuberculosis. Korean J Dermatol. 1985. 23:321–330.
5. Choi SJ, Rho NK, Lee DY, Lee ES. A case of tuberculosis cutis orificialis. Korean J Dermatol. 2002. 40:1293–1295.
6. Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004. 120:305–315.
7. Ichihashi K, Katoh N, Takenaka H, Kishimoto S. Orificial tuberculosis: presenting as a refractory perianal ulcer. Acta Derm Venereol. 2004. 84:331–332.

8. Akgun E, Tekin F, Ersin S, Osmanoglu H. Isolated perianal tuberculosis. Neth J Med. 2005. 63:115–117.
9. Nachbar F, Classen V, Nachbar T, Meurer M, Schirren CG, Degitz K. Orificial tuberculosis: detection by polymerase chain reaction. Br J Dermatol. 1996. 135:106–109.

10. Honig E, van der Meijden WI, Groeninx van Zoelen EC, De Waard-van der Spek FB. Perianal ulceration: a rare manifestation of tuberculosis. Br J Dermatol. 2000. 142:186–187.

11. Schluger NW, Burzynski J. Tuberculosis and HIV infection: epidemiology, immunology, and treatment. HIV Clin Trials. 2001. 2:356–365.

12. Ghiya R, Sharma A, Marfatia YS. Perianal ulcer as a marker of tuberculosis in the HIV infected. Indian J Dermatol Venereol Leprol. 2008. 74:386–388.

13. Lin CY, Yeh SP, Huang HH, Liao YM, Chiu CF. Perianal tuberculosis during neutropenia: a rare case report and review of literature. Ann Hematol. 2006. 85:547–548.

14. Candela F, Serrano P, Arriero JM, Teruel A, Reyes D, Calpena R. Perianal disease of tuberculous origin: report of a case and review of the literature. Dis Colon Rectum. 1999. 42:110–112.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download