Abstract
Aneurysmal benign fibrous histiocytoma is an uncommon pathologic variant of dermatofibroma. In addition to the features of a typical dermatofibroma, it has large cleft-like or cavernous blood-filled spaces with numerous hemosiderin pigments. It should be differentiated from angiomatoid malignant fibrous histiocytoma, malignant melanoma, and vascular tumors such as Kaposi's sarcoma and angiosarcoma. Atrophic dermatofibroma is also a rare variant of dermatofibroma, and the combination of aneurysmal and atrophic features is rarer still. We report a case of aneurysmal benign fibrous histiocytoma with atrophic features in a 27-year-old male who had a grayish-brown atrophic patchy lesion on his back for 2 years.
Benign fibrous histiocytoma (BFH), also known as dermatofibroma (DF), is a common benign fibrohistiocytic lesion that usually occurs as a solitary nodule on the limbs of young to middle-aged adults. It has many clinicopathological variants and the diagnosis may therefore be difficult. Aneurysmal BFH is an uncommon histologic variant of BFH. It is usually larger than typical BFH and sometimes shows rapid growth. Atrophic DF is a rare variant of DF and presents as an atrophic or depressed patch with decreased thickness of the dermis, which is less than 50% of the surrounding dermis. We report a rare and interesting case that has both of these variant features.
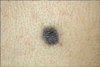
A 27-year-old man visited our clinic with a 2-year history of a brownish patch on his back. He had swelling and pain of this lesion 1 year ago, but recently had no other symptoms. The patient had no specific past medical history or any pertinent family history, and there was no history of trauma. Physical examination revealed a non-tender, fingernail-sized, oval, grayish-brown, atrophic, shiny patch on the upper back (Fig. 1). Clinically, the lesion was initially thought to be a malignant melanoma or an inflamed seborrheic keratosis, and a skin biopsy was taken from the center of the lesion.
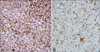
Histopathological findings revealed a non-encapsulated ill-defined mass with numerous large pigments in the dermis without significant epidermal changes. These pigments were positively stained for iron; hence they were hemosiderin pigments (Fig. 2). Multiple cavernous angiomatoid areas were surrounded by histiocytes, fibroblasts, pigments, and capillaries. Extravasated red blood cells and multinucleated giant cells were also observed (Fig. 3). Immunohistochemical analysis showed that the tumor cells were strongly positive for vimentin, negative for S-100, HMB-45, CD68, and factor XIIIa, and focally positive but mainly negative for CD34 (Fig. 4). Considering both clinical and pathologic findings, the final diagnosis of aneurysmal BFH with atrophic features was established. The lesion was completely excised and there was no evidence of recurrence within 5 months after the removal.
BFH usually presents as single or multiple firm reddish-brown nodules located on the lower extremities of young adults. Histopathological findings show a tumor mass in the dermis that is composed of fibroblast-like spindle cells, histiocytes, and blood vessels.
Aneurysmal benign fibrous histiocytoma (ABFH) is an uncommon variant of BFH that was first described by Santa Cruz and Kyriakos in 19811. When compared with typical BFH clinically, the ABFH may be a larger lesion and may also be associated with symptoms of rapid growth and pain. It also has a higher local recurrence rate after removal of the lesion. According to a previous report, about 20% of ABFH lesions undergo abrupt or recent color changes2. In addition to the typical histopathological features of BFH, ABFH has findings such as collections of capillaries, hemorrhagic foci, and hemosiderin deposits around cleft-like and cavernous blood-filled spaces in the center of the tumor. ABFH is reported to be negative for immunohistochemical staining for factor XIIIa, CD34, CD31, CD68, and desmin, but strongly positive for vimentin2.
Because of its diverse histologic appearance, ABFH is frequently confused with other fibrous or vascular tumors such as dermatofibrosarcoma protuberans (DFSP), spindle cell hemangioendothelioma, nodular Kaposi's sarcoma, and angiosarcoma. However, unlike DFSP, ABFH rarely invades the subcutaneous fatty layer and does not have a reticular pattern when it does invade the fatty layer. Moreover, DFSP tumor cells are mostly positive (92%) for CD34 immunohistochemically, while only a small portion of ABFH tumor cells are positive (42%) for CD343. In Kaposi's sarcoma, enlarged cystic structures are surrounded by the endothelium, and the tumor cells are positive for CD34 by immunohistochemical analysis. Angiosarcoma shows atypical proliferation of the endothelium, which is not observed in ABFH.
The exact pathogenesis of ABFH is not clear and remains controversial. There is no proven correlation between external trauma and the hemorrhagic features within the tissues4. Santa Cruz and Kyriakos1 reported that, in some cases of DF, blood slowly extravasates from the capillaries of the tumor and then produces hemosiderin pigments. This continuous extravasation of blood then supposedly fills the tissue spaces and finally forms cavernous or cleft-like areas that are characteristic of ABFH.
Atrophic DF was first described by Page and Assaad5 in 1987, who reported five cases of depressed or atrophic DF and DFSP. Atrophic DF represents about 2% of all DF and commonly occurs on the upper trunk of women. Atrophic DF appears more frequently at older ages compared with typical DF (49 years vs. 20~25 years of age) and has a higher female dominance6. While DF usually presents as firm reddish-brown papules with diameters of 0.5 cm to 1.0 cm, atrophic DF appears as an anetoderma, or depressed plaque. Histologically, atrophic DF is accompanied by dermal atrophy of more than 50% of the locoregional dermis7.
The exact cause of atrophic DF has been under debate. Kiyohara et al8 proposed that this disease might be caused by abnormal elastophagocytosis of collagen fibers by the cells of DF. On the other hand, Beer et al9 insisted that atrophic DF is an unusual type of late-stage DF because the mean age of atrophic DF is older than that of typical DF. Other diseases such as anetoderma, atrophic scar, morphea, sclerosing basal cell carcinoma, localized lipoatrophy, and steroid atrophy must be considered in differential diagnosis.
Our case displays aneurysmal characteristics with atrophy, which are both rare variants of DF. There is only one reported case similar to this case6. However, compared to the previous case, there was no loss of dermal tissue in our case and we suggest that "DF with atrophic features" is a more appropriate description of the present case than "atrophic DF". Two cases of DF with atrophic features have been reported in Korea, but these seem to have resulted from biopsies of an early-stage DF because of the short duration of the disease10. Herein we report a rare and interesting case of variant DF with both aneurysmal and atrophic characteristics, which is extremely difficult to diagnose clinically as a DF.
Figures and Tables
Fig. 2
(A) Histopathological findings revealed a non-encapsulated, ill-defined mass with numerous large pigments in the dermis without significant epidermal changes (H&E, ×40). (B) Multiple cavernous angiomatoid areas were surrounded by histiocytes, fibroblasts, pigments, and capillaries. Extravasated red blood cells and multinucleated giant cells were also observed (H&E, ×200).
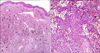
Fig. 4
(A) Immunohistochemical staining was performed using the avidin-biotin complex method. Most of the tumor cells are strongly positive for vimentin (×200). (B) Endothelial cells lining the cavernous spaces and tumor cells focally stain for CD34. However, the majority of tumor cells are negative for DC34 (×100).
References
1. Santa Cruz DJ, Kyriakos M. Aneurysmal ("angiomatoid") fibrous histiocytoma of the skin. Cancer. 1981. 47:2053–2061.

2. Calonje E, Fletcher CD. Aneurysmal benign fibrous histiocytoma: clinicopathological analysis of 40 cases of a tumour frequently misdiagnosed as a vascular neoplasm. Histopathology. 1995. 26:323–331.

3. Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathol. 1997. 19:147–153.

4. Zelger BW, Zelger BG, Steiner H, Ofner D. Aneurysmal and haemangiopericytoma-like fibrous histiocytoma. J Clin Pathol. 1996. 49:313–318.

5. Page EH, Assaad DM. Atrophic dermatofibroma and dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1987. 17:947–950.

6. Curco N, Pagerols X, Garcia M, Tarroch X, Vives P. Atrophic dermatofibroma accompanied by aneurysmatic characteristics. J Eur Acad Dermatol Venereol. 2006. 20:331–333.

7. Zelger BW, Ofner D, Zelger BG. Atrophic variants of dermatofibroma and dermatofibrosarcoma protuberans. Histopathology. 1995. 26:519–527.

8. Kiyohara T, Kumakiri M, Kobayashi H, Ohkawara A, Lao LM. Atrophic dermatofibroma. Elastophagocytosis by the tumor cells. J Cutan Pathol. 2000. 27:312–315.

9. Beer M, Eckert F, Schmoeckel C. The atrophic dermatofibroma. J Am Acad Dermatol. 1991. 25:1081–1082.

10. Kim EH, Kang HY, Lee ES, Kim YC. Two cases of dermatofibroma with atrophic features. Korean J Dermatol. 2007. 45:305–308.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download