Abstract
Background
Ionotropic glutamate receptors of the N-methyl-D-aspartate receptor (NMDAR) type are expressed on keratinocytes and play a role in the proliferation, differentiation, and cornification of keratinocytes. However, the expression profile of NMDAR and its role in cutaneous malignancy is unclear.
Objective
We analyzed the expression of NMDAR-1 in cutaneous squamous cell carcinoma (SCC) and investigated the relationship between NMDAR-1 expression and clinicopathological parameters.
Methods
Thirty-two patients with biopsy-proven cutaneous SCC were enrolled in this study. Each patient was analyzed for tumor diameter, location, local recurrence, and metastasis by conducting a chart review. The SCC specimens were histologically divided into differentiated and undifferentiated groups based on Broders' system. NMDAR-1 expression was examined by performing immunohistochemistry, and the relative staining intensity in the SCCs was graded into 5 levels. According to the staining intensity of NMDAR-1, the specimens were categorized into two groups: the higher group and the lower group.
Results
Fifteen (88%) of 17 tumors in the higher group were differentiated SCC, whereas 14 (93%) of 15 tumors in the lower group were undifferentiated SCC. In addition, NMDAR-1 expression was inversely correlated with metastasis (p=0.049). Local recurrence was associated with a lower staining intensity, but the results were not statistically significant.
Glutamate is an essential amino acid and neurotransmitter in the central nervous system (CNS). It plays a major role in excitotoxicity as well as in learning and memory. In addition, it functions as a trophic factor for cancer cells in vitro1, suggesting an involvement of glutamate and its receptors in tumor cell proliferation and migration. Glutamate activates various glutamate receptors, including N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid, kainite, and metabotropic receptors2,3. NMDAR has two distinct subunits: the main subunit, NMDAR-1, and the modulatory subunit, NMDAR 2A-2D. NMDAR-1 is essential and sufficient to form an active NMDA receptor channel, in contrast to the NMDAR-2 subunits4. The NMDAR is a ligand-gated ion-channel that is permeable to the cations Na+, K+, and most importantly, Ca2+.
Although the expression of the glutamate receptor was initially restricted to the CNS, glutamate signaling occurs in non-neuronal tissues such as bone, pancreas, and skin, raising the possibility of a more widespread glutamate receptor activity5. Moreover, NMDAR-1 is expressed on keratinocytes6. NMDAR-1 expression increased with keratinocytic differentiation in the epidermis of normal human skin and was most intense in the granular cell layers7. This receptor distribution correlates well with the intraepidermal calcium gradient in the normal epidermis8. The regulation of the keratinocytic calcium by NMDAR is important for epidermal differentiation and cornification9. These results suggest that NMDAR could play a role in the proliferation and differentiation of normal keratinocytes. In addition, the presence of NMDAR and its involvement in cellular proliferation are well-known factors in tumor cells derived from neuronal tissue such as glioma and neuroblastoma10,11. However, not much is known about the NMDAR expression and activity in other types of tumors.
Squamous cell carcinoma is one of the most common cutaneous malignancies, with an increasing incidence. The clinical outcome and prognosis of cutaneous SCC depends on many factors, such as tumor size, depth of invasion, and most importantly, the degree of differentiation of the tumor cells12. Little is known about the expression profiles of NMDAR and its role in cutaneous SCC. Here we investigated the expression profile of NMDAR-1 with regard to the histological differentiation and clinical parameters of cutaneous SCC.
Thirty-two patients with biopsy-proven cutaneous SCC were examined in this study. The patients underwent tumor resection surgery between 1998 and 2003 at the Department of Dermatology, Kangnam St. Mary's Hospital, Seoul, Korea. The clinical data, including age, gender, location, tumor size, local recurrence and metastasis, were reviewed from the clinical records.
The samples obtained from surgery were fixed in 4% paraformaldehyde, washed in tap water, dehydrated in a graded series of ethanol solutions, cleared in xylene, and then embedded in paraffin. 4-µm thick sections were mounted on glass slides, hydrated with distilled water and subjected to routine hematoxylin-eosin staining. Broders' system was used to histologically classify conventional cutaneous SCC by grades 1 to 4 on the basis of the degree of nuclear polymorphism and the cytoplasmic maturation to keratinization12. Because many dermatopathologists generally use a variation of this system for investigating the prognostic factors of SCC5,7, the presented cases were divided into two subtypes as follows: differentiated SCC with differentiated lesions in about three-fourths or one half of its structure (Broders' grades 1 and 2), and undifferentiated SCC with undifferentiated lesions in about three-fourths of its structure and with no tendency for differentiation (Broders' grades 3 and 4).
Immunohistochemical staining of NMDAR-1 was performed to determine its expression profile in cutaneous SCC. For each sample, paraffin-embedded specimens fixed in 10% formalin were sectioned at 4 µm thickness, mounted on poly-L-lysine-coated glass slides, and deparaffinized with xylene and a graduated series of ethanol solutions. Subsequently, the sections underwent antigen retrieval via microwave treatment in 10 mM citrate buffer (pH 6.0) for 15 minutes prior to immunostaining. Immunohistochemical staining was performed using the avidin-biotin peroxidase technique13. After soaking in phosphate-buffered saline (PBS) and subsequent blocking of the endogenous peroxidase in 3% hydrogen peroxide for 10 minutes at room temperature, the sections were incubated at room temperature for 1 h with anti-human rabbit primary polyclonal antibodies (1:100 in PBS) against NMDAR-1 (Chemicon, Temecula, CA, USA). Afterwards, the tissue sections were incubated with secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) (1:300 in PBS) for 60 min. As a negative control, skin samples were incubated with PBS without the primary antibodies. The color reaction of the treated tissue was performed using the avidin biotin complex substrate 3-amino-9-ethylcarbazole (Vector Laboratories, Burlingame, CA, USA) for 10 min. The sections were then counterstained with hematoxylin (Vector Laboratories) for 1 to 2 min.
By comparing the stratum granulosum of the surrounding normal epidermis in the same section, which was used as the positive control, the relative staining intensity of NMDAR-1 in the SCC was graded as follows: -: completely negative staining intensity, ±: lower staining intensity, +: more or less overlapped staining intensity in the differentiated areas, ++: slightly higher staining intensity, and +++: considerably higher staining intensity. According to the staining intensity of NMDAR-1, the specimens were categorized into two groups: the higher group, which showed equal or stronger staining than the normal epidermis; and the lower group which showed a completely negative to weaker staining than the normal epidermis. Those samples showing more or less overlapped staining in the differentiated areas of the tumor were categorized as the higher group. Evaluation of the immunohistochemical grading and categorization was done by at least two independent dermatologists who had no knowledge of the clinicopathologic data.
Immortalized human keratinocytes (HaCaT cells) were cultured in a monolayer at 37℃ in 5% CO2 using Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 10,000 units/ml penicillin, and 10,000 g/ml streptomycin (Gibco, Grand Island, NY, USA). All were seeded in a 100 mm culture dish and cells at 80% confluency were subcultured.
HaCaT cells (1×105/well) were seeded in triplicate onto 6-well culture plates (Nunc, Naperville, IL, USA) in 3 ml of medium, cultured, and adhered overnight. The cells were washed and then lysed by incubation for 30 min on ice with radioimmunoprecipitation assay buffer. After sonication, the lysates were centrifuged (RPM 13,000 for 30 min at 4℃), and supernatants were obtained. The protein contents were quantified by the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). Equal amounts of protein (20 g) were separated by electrophoresis on a 4~12% bis-tris gel, and then transferred to a polyvinylidene difluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA). Blots were blocked for 2 h at room temperature with 5% skimmed milk powder in Tris-Buffered Saline Tween-20. The membranes were incubated overnight at 4℃ with primary antibodies. Rabbit polyclonal antibodies against β-actin (1:5000 dilution, Bethyl Laboratories, Inc., Montgomery, TX, USA) and NMDAR1 (1:500 dilution, Chemicon) were used in this study. The blots were then incubated with the corresponding conjugated anti-rabbit immunoglobulin G-horseradish peroxidase (1:1000 dilution, R&D Systems, Inc., Minneapolis, MN, USA). Immunoreactive proteins were visualized by enhanced chemiluminescence (Amersham Biosciences, Buckinghamshire, UK).
Pearson's correlation analyses were performed to examine whether NMDAR-1 expression of cutaneous SCC was related to clinicopathological parameters such as the histological differentiation, local recurrence, and metastasis rate for each patient. The data was analyzed using SPSS for Windows 10.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as p<0.05.
The patients' profiles are summarized in Table 1. The examined patients consisted of 11 males and 21 females, with ages ranging from 41 to 94 years old. The tumor diameter ranged from 7 to 150 mm. Three of the examined patients had a local recurrence (9.4%) and 6 had metastatic disease (18.8%); all 6 metastasizing SCCs were over 3 cm in diameter.
To examine the specificity of the anti-NMDAR-1 antibody, we first performed Western blotting for NMDAR-1 in cultured human keratinocytes (Fig. 1). All of the surrounding normal epidermis of the SCC specimens commonly showed a distribution pattern with maximum NMDAR-1 intensity in the stratum granulosum (with a gradient that corresponded to increasing differentiation) (Fig. 2A, B). Depending on the relative intensity of NMDAR-1 expression, the specimens were categorized into two groups: higher expression in 17 specimens (53%, the higher group) and lower expression in 15 specimens (47%, the lower group) compared with the stratum granulosum of the normal epidermis in the same sections. Six specimens in the higher group exhibited a considerably higher expression of NMDAR-1 in all tumor cells (Fig. 2C, D), and the other 7 specimens exhibited equal or slightly higher levels of expression in the tumor region compared with the normal epidermis. All of the 4 overlapped specimens in the higher group showed a high expression of NMDAR-1 that was restricted to the differentiated areas of the tumor (Fig. 2E, F). In contrast, 4 specimens in the lower group exhibited a lower expression of NMDAR-1, and the remaining 11 specimens were completely negative for any NMDAR-1 expression (Fig. 2G, H).
When both the higher and lower groups were categorized by histologic differentiation, 15 (88%) of 17 tumors in the higher group were classified as differentiated SCC, whereas 14 (93%) of 15 tumors in the lower group were undifferentiated SCC (Table 2), suggesting that there was a significant correlation between NMDAR-1 expression and histological grading in cutaneous SCC (p<0.0001). We then examined the relationship between NMDAR-1 expression and other clinical parameters such as local recurrence and metastasis. Among the three patients with local recurrence, one was in the higher group of NMDAR-1 expression, whereas the other two were in the lower group. Among the six patients with metastasis, one was in the higher group of NMDAR-1 expression, whereas the other five were in the lower group (Table 2).
Pearson's correlation analysis revealed that the lower expression of NMDAR-1 was significantly correlated with a higher frequency of metastasis (33% vs 6%, respectively, for the two groups) (p<0.0487). Local recurrence in the group with a lower expression of NMDAR-1 (13%) was more frequent than in the group with the higher expression of NMDAR-1 (6%), but there was no statistical significance due to the small number of patients with local recurrence.
We investigated the NMDAR-1 expression profile in cutaneous SCC and demonstrated that the intensity of NMDAR-1 expression has a tendency to depend on the histological differentiation, as seen in NMDAR-1 expression of normal skin. We showed that most (88%) of the higher NMDAR-1 expression group was differentiated SCC, whereas most (93%) of the lower NMDAR-1 expression group was undifferentiated.
One previous study examined NMDAR-1 expression in patients with oral SCC, with positive NMDAR-1 reactivity in 50 of 81 cases14. However, in their study, NMDAR-1 expression was simply defined as being negative if the positive cells comprised <25%, and positive when the proportion of positive cells comprised >25%. Furthermore, they did not categorize the tumor specimens according to tumor differentiation, as was done in our study. Considering the fact that oral mucosal epithelium does not cornify and that the regulation of the keratinocytic calcium by NMDARs is especially important in the cornifying process, it is not reasonable to directly compare the NMDAR-1 expression pattern of oral SCC with that of cutaneous SCC.
Another study examined the expression pattern of NMDAR-1 in primary cutaneous SCC6. The results showed that the reactive epithelium (non-neoplastic) surrounding the SCC showed strong NMDAR-1 expression, but the SCC itself did not express NMDAR-1. NMDAR may play a role in the contact-mediated inhibition of growth that is lacking in most cutaneous neoplastic processes. However, because the degree of differentiation and the number of specimens was not shown, the exact expression pattern of NMDAR-1 in cutaneous SCC could not be inferred from their study. Therefore, this is the first report to show such a distinctive expression pattern of NMDAR-1 in cutaneous SCC according to tumor differentiation. Of particular interest, the expression of NMDAR-1 in cutaneous SCC was well correlated with the degree of differentiation of the tumor cells. Selective blockade of NMDAR-1 with MK-801 suppresses the expression of filaggrin and CK10 as differentiation markers in HaCaT cells, but this had no influence on the proliferation of HaCaT cells7. This suggests that NMDAR-1 in normal skin plays a role in the differentiation of the epidermis, rather than the proliferation. Moreover, although the role of NMDAR-1 in non-cutaneous cancers such as neuroblastoma10 is mainly focused on tumor proliferation, it appears that NMDAR-1 in cutaneous SCC affects differentiation. Although the molecular mechanism underlying the close correlation of NMDAR-1 expression and the histological differentiation in cutaneous SCC was not elucidated in this study, higher NMDAR-1 expression could suppress advances in transformation, whereas lower expression induces dedifferentiation of tumor cells. Further study will be needed to clarify the role of NMDAR-1 expression in the differentiation of cutaneous SCC.
Several prognostic factors, such as location, size, tumor thickness, histologic differentiation, local recurrence, and metastasis could be significantly involved in the survival of patients with SCC of the skin15-20. For example, an increased diameter of cutaneous SCC is associated with an increased metastatic rate. The majority of metastasizing cutaneous SCCs are greater than 2 cm in diameter, and many recurrent cutaneous SCCs are greater than 1 cm in diameter20,21. In this study, all 6 metastasizing SCCs were over 2 cm in diameter. Moreover, Pearson's correlation analysis revealed that the number of patients with metastasis in the lower NMDAR-1 expression group was more than that in the higher expression group (p<0.05). Although the number of patients with local recurrence was too small for proper statistical analysis, the rate of local recurrence of the lower expression group was higher than that of the higher expression group. Therefore, NMDAR-1 could be an indicator for the prognosis of cutaneous SCC.
In conclusion, our data demonstrate that the expression pattern of NMDAR-1 is well correlated with histologic differentiation in cutaneous SCC. We also suggest NMDAR-1 is a prognostic indicator for cutaneous SCC.
Figures and Tables
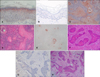 | Fig. 2The expression of NMDAR-1 in cutaneous SCC. (A) Immunohistochemistry of NMDAR-1 in the surrounding normal epidermis of the SCC specimen used as the positive control, (B) Negative control; ×100, (C, D) Differentiated SCC with considerably higher expression of NMDAR-1, (E, F) Well- to poorly differentiated SCC with localized expression of NMDAR-1 in differentiated areas, (G, H) Undifferentiated SCC with completely negative expression of NMDAR-1 (C, E, G: Immunohistochemical staining for NMDAR-1; D, F, H: H&E, ×100). |
References
1. Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proc Natl Acad Sci U S A. 2001. 98:6372–6377.

2. Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000. 130:1007S–1015S.

3. Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981. 21:165–204.

4. Foldes RL, Rampersad V, Kamboj RK. Cloning and sequence analysis of cDNAs encoding human hippocampus N-methyl-D-aspartate receptor subunits: evidence for alternative RNA splicing. Gene. 1993. 131:293–298.

5. Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001. 22:174–181.

6. Nahm WK, Philpot BD, Adams MM, Badiavas EV, Zhou LH, Butmarc J, et al. Significance of N-methyl-D-aspartate (NMDA) receptor-mediated signaling in human keratinocytes. J Cell Physiol. 2004. 200:309–317.

7. Fischer M, Glanz D, William T, Klapperstuck T, Wohlrab J, Marsch W. N-methyl-D-aspartate receptors influence the intracellular calcium concentration of keratinocytes. Exp Dermatol. 2004. 13:512–519.

8. Menon GK, Elias PM. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol. 1991. 127:57–63.

9. Fischer M, William T, Helmbold P, Wohlrab J, Marsch W. Expression of epidermal N-methyl-D-aspartate receptors (NMDAR1) depends on formation of the granular layer--analysis in diseases with parakeratotic cornification. Arch Dermatol Res. 2004. 296:157–162.

10. North WG, Fay MJ, Du J, Cleary M, Gallagher JD, McCann FV. Presence of functional NMDA receptors in a human neuroblastoma cell line. Mol Chem Neuropathol. 1997. 30:77–94.

11. Yoshioka A, Ikegaki N, Williams M, Pleasure D. Expression of N-methyl-D-aspartate (NMDA) and non-NMDA glutamate receptor genes in neuroblastoma, medulloblastoma, and other cells lines. J Neurosci Res. 1996. 46:164–178.
12. Broders AC. Squamous-cell epithelioma of the skin: a study of 256 cases. Ann Surg. 1921. 73:141–160.

13. Maatkamp A, Vlug A, Haasdijk E, Troost D, French PJ, Jaarsma D. Decrease of Hsp25 protein expression precedes degeneration of motoneurons in ALS-SOD1 mice. Eur J Neurosci. 2004. 20:14–28.

14. Choi SW, Park SY, Hong SP, Pai H, Choi JY, Kim SG. The expression of NMDA receptor 1 is associated with clinicopathological parameters and prognosis in the oral squamous cell carcinoma. J Oral Pathol Med. 2004. 33:533–537.

15. Barksdale SK, O'Connor N, Barnhill R. Prognostic factors for cutaneous squamous cell and basal cell carcinoma. Determinants of risk of recurrence, metastasis, and development of subsequent skin cancers. Surg Oncol Clin N Am. 1997. 6:625–638.

16. Johnson TM, Rowe DE, Nelson BR, Swanson NA. Squamous cell carcinoma of the skin (excluding lip and oral mucosa). J Am Acad Dermatol. 1992. 26:467–484.

17. Kwa RE, Campana K, Moy RL. Biology of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992. 26:1–26.

18. Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992. 26:976–990.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download