Abstract
Background
Radiation therapy (RT) including tomotherapy has been widely used to treat primary tumors, as well as to alleviate the symptoms of metastatic cancers.
Objective
The
primary purpose of this study was to examine the characteristics of the clinical features and pathophysiological mechanisms associated with acute radiation dermatitis in cancer patients that received tomotherapy, and compare the results to patients treated by conventional radiation therapy.
Methods
The study population consisted of 11 patients that were referred to the dermatology department because of radiation dermatitis after receiving tomotherapy; all patients were evaluated for clinical severity. The patients were assessed and identified using the National Cancer Institute Common Toxicity Criteria version (CTC) 3.0. We performed biopsies of the skin lesions that were examined for apoptosis using the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labelling (TUNEL) assay and stained immunohistochemically with monoclonal antibodies to CD8, CD4 and TGF-β. As a positive control, patients with radiation dermatitis treated with conventional radiation therapy were also studied.
Results
The results of the
clinical features of the skin of tomotherapy patients were the following: grade 1 (36%), grade 2 (55%) and other changes (9%). Among the population that had skin lesions due to acute radiation dermatitis, the mean number of positive cells per high power field (HPF) was the following: there were 30.50±7.50 TUNEL-positive cells, 34.60±12.50 CD8+ T cells, 5.19±3.17 CD4+ T cells and 9.95±1.33 TGF-β positive cells measured per HPF. The mean number of positive cells per HPF for the patients that received conventional radiation therapy was: TUNLEL-positive cells in 7.5±1.64, CD8-, CD4- and TGF-β-positive cells in 12.50±3.73, 3.16±1.47, 6.50±1.97.
Conclusion
We found that the number of TUNEL-positive cells and CD8+ T cells were higher in the lesions of patients receiving tomotherapy compared to the lesions of the patients receiving conventional radiation therapy. These findings suggest that tomotherapy without dose modification may cause significantly more severe forms of radiation dermatitis by apoptosis and cytotoxic immune responses than conventional radiation therapy.
Recent advances in medical science, especially the development of molecular biological techniques and medical technology, have contributed to the improved therapeutic efficacy of cancer treatment protocols. Radiation therapy (RT), as monotherapy or in combination with other treatment modalities, has been reported as an effective regimen for tumor treatment. However, use of traditional RT, to treat tumors, also affects normal tissues adjacent to the cancer1. By contrast, tomotherapy is a more advanced form of conformal RT2. With tomotherapy, the intensity of the beam varies across the aperture to shape the dose distributions around targets in a way that is not possible with conventional techniques3. Radiation-induced skin changes have been recognized since the discovery of X-rays by Roentgen in 1895; these harmful effects were first reported in 19021,4. The total dose, dose/fraction, type and quality of the beam, and the volume and surface area exposed all influence the degree of damage to the skin5. Clinical and microscopic changes of the skin develop after therapeutic exposure to radiation. Within two to three weeks after exposure to radiation, erythema, desquamation and erosion occur. In acute radiation dermatitis, there is edema, endothelial cell changes and other epidermal and dermal cell changes such as inflammation, cell apoptosis and necrosis by lymphocytes and cytokines1. Cell death usually occurs after one to five radiation division cycles. Radiation damage to chromosomal DNA is responsible for cell destruction, such as apoptosis, necrosis, and failure to complete mitosis6. The later post-radiation changes result from injury to the vasculature and fibrosis, which is cytokine-mediated. Transforming growth factor-β (TGF-β) is associated with fibrosis. In addition, RT may have a negative impact on long-term cosmetic outcome as well as acute and chronic radiation dermatitis. These complications are closely related to a decreased quality of life after RT3,7. Therefore, the primary purpose of this study was to determine the characteristics of the clinical features associated with acute radiation dermatitis. The severity of acute radiation dermatitis after receiving tomotherapy was examined both clinically and histopathologically, and compared with conventional radiation dermatitis.
The study population consisted of ten women and one man (mean age, 54 years) that received RT at Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea from June 2006 to June 2007 (Table 1). The inclusion criteria were patients that received tomotherapy (Helical Tomotherapy, Hi-Art®, Tomotherapy incorporated. USA). The patients underwent simulation with computed tomography and were prescribed standard fractionation with 180 cGy per day to a total of 4,500 to 5,940 cGy over 28 treatments with tomotherapy. The treatments took 36 to 79 days; patients that experienced radiotherapeutic complications rested between the tomotherapy sessions.
The patient demographics, tumor characteristics and treatment-related information were prospectively collected from the hospital database. We used the National Cancer Institute Common Toxicity Criteria, version (CTC) 3.0, to evaluate the clinical severity of the acute radiation dermatitis1. Grade 1 changes included faint erythema and dry desquamation. Grade 2 changes consisted of persistent tender or edematous erythema that in some cases progressed to focal loss of the epidermis and moist desquamation in the skin folds. Grade 3 dermatitis was characterized by confluent moist desquamation in locations other than the skin folds and pitting edema. Grade 4 changes could progress to ulceration and bleeding1. The treatment protocol for each patient was continued until the study was terminated. Patients were instructed to apply an emollient and topical corticosteroid twice a day and a saline wound dressing daily to the irradiated field after the development of radiation dermatitis. A physician evaluated the degree of skin toxicity every week during the RT in the outpatient clinic. A flow sheet was used for all patients to record the maximum skin toxicity over time, including the scores.
Eleven patients, who understood and agreed to the purpose of the study, were enrolled and provided informed consent to participate. Skin samples were taken at the first visit to our department from patients that had grade 1~2 radiation dermatitis that occurred after undergoing 6~49 days of tomotherapy (Table 1). Three biopsy specimens were taken from each patient. Skin samples from positive controls were taken from three patients with grade 1~2 radiation dermatitis that occurred after 2~4 weeks of treatment with conventional RT. As a negative control, normal skin samples from the three normal subjects were obtained.
This study was conducted in accordance with applicable good clinical practice guidelines and in accordance with the ethical principles for protection of human rights described in the Declaration of Helsinki. The original protocol and the patient consent form were approved by the Institutional Review Boards of Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea.
DNA strand breaks associated with an apoptotic response can be identified by labeling the free 3'-OH termini with modified nucleotides in an enzymatic reaction. Based on the principle of the TUNEL assay, apoptosis in the radiation dermatitis lesions was examined using an in situ cell death detection kit (CHEMICON, S7100, USA). Formalin-fixed tissues were embedded in paraffin and were then sectioned at a 5 µm thickness. The tissue sections were mounted on slides and were deparaffinized, diluted proteinase K was added, and the sections were blocked with 3% H2O2 in PBS. The sections were then treated with the TUNEL reaction mixture composed of terminal deoxynucleotidyl transferase, followed by incubation with a color antibody diaminobenzidine solution.
Immunohistochemical staining for CD8, CD4 and TGF-β was performed to identify the cells that were possible biological markers of radiation dermatitis. Immunohistochemical analysis was performed using a high temperature antigen unmasking technique. The sections were heated in the unmasking solution (citrate buffer), washed, and then were incubated with mouse primary monoclonal antibodies against CD8 (NCL-L-CD8-295; Novocastra Laboratories, Newcastle upon Tyne, UK), CD4 (NCL-L-CD4-1F6; Novocastra Laboratories) and TGF-β (NCL-TGF-β, Novocastra Laboratories) at room temperature for 60 min. This procedure was followed by incubation with the secondary antibodies (Envision Detection kit K5007, DAKO, Denmark). These reaction products were developed using diaminobenzidine solution as a chromogen.
Staining patterns on slides were evaluated under a conventional light microscope at a magnification of ×400. Positively stained cells in the epidermis and upper dermis, regardless of the staining intensity, were counted for each marker on two high marked fields per specimen. Two independent dermatologists reviewed the results in a blinded fashion. Data were analyzed by determination of mean values. Differences between the groups were compared by the Kruskal-Wallis test.
The patient results in the tomotherapy group are summarized in Table 1. The maximum skin toxicities were examined weekly. According to the CTC, the skin toxicities among the 11 tomotherapy patients included 36% that were grade 1 and 55% were grade 2 (Fig. 1). One patient presented only with post-inflammatory hyperpigmentation. No patient had a grade 3 or higher toxicity. Grade 2 changes, consisting of moderate erythema and moist desquamation, occurred 2~4 weeks after radiation therapy. Faint erythema or pigmentation remained 5~7 weeks after the radiation therapy. Patients reported more discomfort with pruritic and burning sensation during the first 1~2 weeks after radiation treatments. Post-inflammatory hyperpigmentation was commonly observed after healing of the acute radiation dermatitis. The radiation dermatitis in most of the patients improved after 2~4 weeks of dermatology care.
The lesions associated with tomotherapy radiation dermatitis were examined for the presence of TUNEL-positive cells (30.50±7.5/HPF). In situ detection of apoptotic cell death by the TUNEL assay showed strong positive signals in the epidermal keratinocytes and dermal endothelial cells as well as inflammatory cells in the lesions (Fig. 2). On the other hand, only faint apoptotic signals were detected in a few cells (7.5±1.64/HPF) in the epidermis and dermis of the skin samples from patients that received conventional radiation therapy (Fig. 2). In addition, only a few cells (2.0±1.65/HPF) in the dermis of normal skin were noted to have faint apoptotic signals. The results showed a more extensive occurrence of apoptosis in the lesions from patients with tomotherapy associated radiation dermatitis compared to conventional radiation therapy lesions by the TUNEL assay (p=0.0019) (Fig. 3).
The inflammatory T-cells that infiltrated into the radiation dermatitis lesions were dominated by CD8+ T cells with a small proportion of CD4+ T cells (Fig. 2). CD8+ T cells in the radiation dermatitis lesions were detected in many of the dermal infiltrates of the tomotherapy patients (34.6±12.5/HPF), whereas they were only weakly detected in the patients receiving conventional radiation therapy (12.50±3.73/HPF) (p<0.0001) (Fig. 2, 3). These cells were rarely observed in normal skin (2.33±0.15/HPF). The CD4+ T cells in the radiation dermatitis lesions were moderately detected in the dermis of tomotherapy patients (5.19±3.17/HPF) and in patients receiving conventional radiation therapy (3.16±1.47/HPF) (p<0.0001) (Fig. 2, 3). However, they were rarely observed in normal skin (0.41±0.9/HPF). The ratio of CD4 to CD8 cells was 40% lower in the dermis of tomotherapy patients than in patients receiving conventional radiation therapy.
The expression of TGF-β in the radiation dermatitis lesions was examined (Fig. 2). Immunohistochemical localization revealed that the presence of TGF-β was detected (9.95±1.33/HPF) in the dermis of lesions associated with tomotherapy radiation dermatitis and in a few cells (6.50±1.97/HPF) in the dermis of patients with conventional radiation dermatitis (Fig. 2). However, the presence of TGF-β was rarely observed in normal skin (0.83±0.52/HPF). The differences in the results among the three groups were statistically significant (p<0.0001) (Fig. 3).
The results of this study showed that 36% and 55% of the patients were classified as grade 1 and grade 2, respectively. A transient, faint grade 1 erythema might develop due to dilatation of capillaries or possibly be associated with increased vascular permeability. Grade 2 changes, consisting of tender and edematous erythema, might occur due to fibrin thrombi or edema of large vessels1,8. Grade 2 erythema may progress to focal loss of the epidermis, producing moist desquamation in the skin folds or creases. The dry desquamation associated with grade 1 is characterized by pruritus and scaling. The moist desquamation of grade 2 is characterized by epidermal necrosis, persistent edema and fibrinous exudates. These erythematous and desquamative reactions after RT are reversible and can heal gradually, even though the reactions are accompanied by increased expression of epidermal growth factor receptors1,9. Complete healing usually occurs one to three months following epidermal regeneration1. In this study, most of the patients reported that the skin lesions were almost completely resolved after two to four weeks of dermatological care, including a wet dressing and topical steroid application. Post-inflammatory hyperpigmentation is commonly observed with any disruption of the dermoepidermal junction, and depends on the severity of the initial reaction and the skin type of the patient6. In this study, the skin type of all the patients was Fitzpatrick III-IV, and faint erythema and pigmentation persisted for five to seven weeks after radiation therapy.
In this study a CTC grade 1 or 2 radiation dermatitis occurred two to four weeks after tomotherapy, with a dose to the skin of 10~50 Gy (mean: 27.9 Gy). On the basis of a recent review, when administering radiation doses to the skin of 40 Gy or greater, grade 2 radiation dermatitis would be expected to occur four to five weeks after therapy8. Grade 2 radiation dermatitis in this study occurred more rapidly than previously reported. There were more grade 2 patients among the cases immediately referred to the dermatology department. The intensity of the radiation dermatitis has been related to age, gender, anatomic site, field size, the radiation dose and the dose rate6,10.
After radiation therapy, free radicals react with the organic molecules of the cells and tissues, and alter their structure and function. Radiation-induced injury occurs instantaneously after exposure, and this injury is mediated by a burst of free radicals that cause DNA damage and alteration of proteins, lipids and carbohydrates. Each additional exposure or fraction contributes to the recruitment of inflammatory cells as well as direct tissue injury11. The activated cell response pathways mediate cytoprotective and cytotoxic responses associated with cell survival or death. Necrosis of cells, failure of cell division and apoptosis are the principal mechanisms of post-radiation cell death. Therefore, acute injury results in the reduction or impairment of stem cell function, endothelial cell changes, as well as inflammation and/or epidermal cell apoptosis or necrosis. Lower radiation doses produce clumping of nuclear chromatin, swelling of the nucleus and apoptosis. Higher doses cause nuclear abnormalities, loss of the nuclear membrane, mitochondrial distortion, degeneration of the endoplasmic reticulum, as well as direct necrosis of cells9,12-14. The results of this study showed an increased frequency of apoptosis by the TUNEL assay in the lesions associated with the tomotherapy radiation dermatitis compared to the conventional radiation dermatitis. In addition, CD4+ T cell and CD8+ T cell mediated inflammation was observed in the lesions. Moreover, T cell mediated inflammation was increased in the lesions of the tomotherapy patients. TGF-β, is a peptide that is involved in mediating the proliferation of many cell types, and it is involved in the development of chronic radiation dermatitis15. Up-regulation of TGF-β has been found in the fibrotic tissue of patients with the chronic effects of radiation; confirming that its induction is a general response of cells to conventional radiation therapy15. TGF-β is also a chemotactic factor for mast cells and possibly increases angiogenesis by inducing macrophages to release factors that lead to neovascularization1,16. In the present study, TGF-β-positive cells were not frequently observed in patients treated with either tomotherapy or conventional radiotherapy, because this study was limited to the effects of acute radiation dermatitis.
One of the advantages of tomotherapy for cancer treatment is its clinical feasibility. More specially, radiation protocols based on individual differences can be developed to improve dose distribution for the treated cancer tissues and lower the dose effects on normal organ tissues compared to more conventional approaches that provide uniform treatment with the same dose of radiation. In addition, there is a low frequency of acute skin toxicity and excellent cosmetic results with tomotherapy3. In this study patients were analyzed that received the same prescribed doses, for both the tomotherapy and conventional patients, for the initial cancer tissue fields and the boost treatments. We have demonstrated the extensive occurrence of apoptosis in the lesions associated with tomotherapy radiation dermatitis using the TUNEL assay. Repetitive radiation exposure leads to a series of skin and soft tissue injury; these consecutive radiation exposures, without regeneration phases, aggravate the existing damage even more.
Although normal skin is not the primary target of ionizing radiation, in most cases, exposure of the normal skin is inevitable. The findings of this study suggest that radiation dermatitis in patients receiving tomotherapy without dose modification had a significantly more severe dermatitis clinically and histologically when compared to patients receiving conventional radiation. The skin of the focal radiation lesions showed apoptosis and CD8+ T cell mediated cytotoxic immune responses.
Figures and Tables
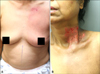 | Fig. 1Clinical photographs of tomotherapy patients. (A) Grade 1 dermatitis with faint erythema on the anterior chest wall. (B) Grade 2 dermatitis with moist desquamation and pigmentation in the neck crease. |
 | Fig. 2Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL)-positive cells and CD8+ T cells were strongly present in the dermal infiltration of the lesions of tomotherapy associated radiation dermatitis, but rarely detected in patients that received conventional radiation therapy; CD4+ T cells and TGF-β stained cells were present in the cells of the dermal infiltrate in the lesions of tomotherapy associated radiation dermatitis and weakly detected in the lesions associated with conventional radiation. |
References
1. Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006. 54:28–46.

2. Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys. 2001. 51:880–914.
3. Freedman GM, Anderson PR, Li J, Eisenberg DF, Hanlon AL, Wang L, et al. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol. 2006. 29:66–70.

4. Frieben H. Demonstration eines Cancroid des rechten Handruckens, das sich nach langdauernder Einwirkung von Rontgenstrahlen entwickelt hatte. Fortschr Rontgenstr. 1902. 6:106–111.
5. Hall EJ, Cox JD. Cox JD, Ang KK, editors. Physical and biological basis of radiation therapy. Radiation oncology. 2003. 8th ed. St. Louis: Mosby;3–62.
6. Frederick DM, Renato GP. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ. Radiobiology and radiation effects. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;858–862.
7. Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, et al. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000. 48:1307–1310.

8. Mendelsohn FA, Divino CM, Reis ED, Kerstein MD. Wound care after radiation therapy. Adv Skin Wound Care. 2002. 15:216–224.

9. Peter RU, Beetz A, Ried C, Michel G, van Beuningen D, Ruzicka T. Increased expression of the epidermal growth factor receptor in human epidermal keratinocytes after exposure to ionizing radiation. Radiat Res. 1993. 136:65–70.

10. James WD, Berger TG, Elston DM. Andrews' diseases of the skin: clinical dermatology. 2006. 10th ed. Philadelphia: Saunders Elsevier;39–41.
11. Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex 'wound'. Radiother Oncol. 2002. 63:129–145.
12. Malkinson FD, Keane JT. Radiobiology of the skin: review of some effects on epidermis and hair. J Invest Dermatol. 1981. 77:133–138.

13. Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990. 57:751–773.

14. Mettler FA, Upton AC. Medical effects of ionizing radiation. 1995. 2nd ed. Philadelphia: W.B. Saunders;14–21.
15. Canney PA, Dean S. Transforming growth factor beta: a promotor of late connective tissue injury following radiotherapy? Br J Radiol. 1990. 63:620–623.

16. Gruber BL, Marchese MJ, Kew RR. Transforming growth factor-beta 1 mediates mast cell chemotaxis. J Immunol. 1994. 152:5860–5867.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download