Abstract
Background
Mucoceles have diverse clinical and histological features. Their pathogenesis remains to be elucidated.
Objective
To determine the structural and/or pathogenic differences between two clinically different types of mucoceles.
Methods
Seventeen oral mucoceles were examined clinically and immunohistologically. The mucoceles were divided into two groups by their clinical manifestations: papular group (PG) and nodular group (NG).
Results
Histologically, granulation tissue formed more frequently in the NG group, while CD4 and CD8 positive cells were more abundant in the PG group. There were no significant differences in the tumor necrosis factor-alpha or the matrix metalloproteinases (MMP)-2 and 9, between the two groups.
Conclusion
There were significant differences in the depth of the lesions, granulation tissue formation and infiltrating T lymphocytes between the PG and NP type of mucoceles. These findings suggest that the clinical manifestations may be influenced by the type of inflammatory response and extracellular matrix remodeling.
An oral mucocele is a benign, painless, dome-shaped, fluctuant cyst that usually occurs inside of the lower lip. In order to determine the frequency of recurrence in susceptible patients and the need for invasive treatments (e.g. surgical removal, laser destruction or cryotherapy), the pathogenesis and precise classification of mucoceles must be clear. Grossly, a mucocele lesion of the lip is typically described as a round or oval dome-shaped, fluctuant, nodular, freely movable swelling. In some cases, a mucocele presents as a horn-shaped, protruding, whitish papule. Despite the differing clinical features, there have been no trials to classify oral mucoceles by their clinical features.
A mucocele is caused by a traumatic rupture of a minor salivary gland duct1. After traumatic rupture and extravasation of mucus, remodeling of the extracellular matrix is the key mechanism underlying the formation of mucoceles2,3. Microscopically, mucoceles can present with a wide range of structural variations. The most common type of mucocele is characterized by the formation of a well-circumscribed, cyst-like space surrounded by granulation tissue with varying degrees of maturity4. By contrast, some specimens consist only of a collapsed wall of granulation tissue that contains muciphages5.
The purpose of this retrospective clinical and pathological review of biopsy specimens from oral mucoceles was to evaluate two groups of mucoceles according to their clinical features, and determine the differences between the two clinical groups with regard to their histological and immunohistochemical features.
Sixteen patients with mucoceles of the oral mucosa were included in this study. We obtained informed consent from each patient before conducting any study procedure. This clinical study was performed in accordance with Good Clinical Practices and the Declaration of Helsinki (2000), and the protocol was approved by the Catholic Medical Center Subcommittee on Human Studies on August 29, 2007.
We examined 17 mucoceles that were surgically removed from 16 patients. The personal and medical histories of the patients were reviewed, including the presence of lip-biting habits, history of oral trauma and the duration and size of the lesions. The size of each mucocele was defined as the diameter at the base of the lesion. Clinical photographs were reviewed by two dermatologists. The mucoceles were classified into two groups according to their clinical manifestations. The papular group (PG) had well-demarcated, superficially located, white, and protruding horn-shaped lesions (Fig. 1A). The nodular group (NG) had indistinct margins, deep, erythematous, and swollen dome-shaped lesions (Fig. 1B).
Excised tissues were fixed in a 5% formalin solution and embedded in paraffin. The tissues were cut into 2 µm sections. The depth of the lesion was measured using two indices. Depth measurement A was defined as the distance from the basal layer of the mucosa to the most superficial margin of a lesion. Depth measurement B was defined as the distance from the basal layer of the mucosa to the deepest margin of a lesion.
Immunohistochemical staining was performed on 2 µm serial sections cut from formalin-fixed and paraffin-embedded archived tissues. Peroxidase-antiperoxidase (PAP) staining was performed. For all cases, we studied the anti-tumor necrosis factor-alpha (TNF-α monoclonal antibody, clone 52B83; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:50), apoptosis using a detection kit (polyclonal antibody, clone 52B83; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:50), anti-matrix metalloproteinase-2 (MMP-2 monoclonal antibody, clone A-GTel VC2; Neomarkers, Fremont, CA, USA; 1:50), anti-MMP-9 (MMP-9 monoclonal antibody, clone 15W2; Novocastra, Newcastle, UK; 1:50), anti-CD4 (CD4 monoclonal antibody, clone 1F6; Novocastra, Newcastle, UK; dilution 1:50), and anti-CD8 (CD8 monoclonal antibody, clone 1A5; Novocastra, Newcastle,UK; dilution 1:50). Visualization of the presence of MMP-2, MMP-9 and TNF-α, was performed with diaminobenzidine (DAB; Dako, Carpinteria, CA, USA) as the chromogen after incubation with antibody for 60 min at room temperature. For the apoptosis detection kit, the specimens were deparaffinized, pre-treated with proteinase K (20 µg/ml), placed in 3% H2O2 for 5 minutes and incubated with TdT enzyme at 37℃ for 1 hour. DAB was used as a chromogen. For the CD4 and CD8 levels, the sections were incubated with each specific antibody for 60 min at room temperature after microwave heating in citrate buffer (pH 6.0); DAB was also used as a chromogen. Immunostained slides were independently evaluated by two dermatologists.
For each immunostained tissue section, the entire tumor area was evaluated for infiltrating lymphocytes and apoptosis at a given scanning power (10×objective and 10×eye piece). Areas with the most abundant staining were selected, and a maximum of six randomly chosen fields were evaluated at high-power (40×objective and 10×eye piece). For all cases, the number of CD4(+), CD8(+) and apoptosis(+) cells were counted. For the assessment of MMP-2, MMP-9 and TNF-α, only the qualitative characteristics (positive or negative) were recorded.
The means±standard deviation. for age, the duration of the lesions and immunostained cell counts were compared between the papular (PG) and nodular (NG) groups with the two-sample t test. The ratios of the formation of well-defined cysts or granulation tissues, and positive results for MMP-2, MMP-9 and TNF-α were analyzed by the Chi-Square test. The significance level was set at p<0.05. The SPSS statistical program was used for the analysis.
Mucoceles were classified into the two aforementioned groups. Eight specimens from seven patients were compatible with the papular group (PG) criteria, and nine specimens from nine patients were classified as the nodular group (NG). One patient had two separate protruding whitish papules at the base of the tongue. Only one patient in each group had a history of a traumatic tear, and two patients in the NG group had histories of habitual lip-biting. The patient age, duration and size of the lesions are summarized in Table 1. There were no statistically significant differences in age or duration between the two groups. The mean size of the lesions in the PG and the NG were 3.13±0.64 mm and 6.67±2.55, respectively. The lesions were larger in the NG group compared to the PG group (p=0.003).
The mucoceles were observed as either well-defined cysts or masses of granulation tissue. For the well-defined cysts, the walls were surrounded by either epithelial cells or thin granulation tissue (Fig. 2A). In contrast to well-defined cysts, some specimens were composed of non-encapsulated granulation tissue. This granulation tissue was composed of widely scattered mucus mixed with vacuolated macrophages, lymphocytes and fibroblasts (Fig. 2B, C). The percent of specimens with granulation tissue as the main pathological lesion was 25% in the PG group and 78% in the NG group. Granulation tissue formation was more frequently observed in the NG group compared to the PG group (p=0.03). For the depth measurements, all specimens in the PG group were available for evaluation, but only six of the nine specimens in the NG were available for evaluation. The other three specimens in the NG group were not available because the specimens were either bent or broken. The mean A depth was 6.25±17.67 µm in the PG group and 282.89±341.22 µm in the NG group. The mean B depth was 1,831.25±818.29 µm in the PG group and 1,935.67±2,207.68 µm in the NG group. For comparison according to the A depth, there was a statistically significant difference between the two groups (p=0.041); however, the B depth comparison, was not statistically significant (p=0.897).
The results of the CD4(+) and CD8(+) infiltrating lymphocytes are summarized in Table 2 and shown in Fig. 3A~D. Mucosal apoptosis was most prominent in the basal layer of the oral mucosa, and it gradually decreased in the upward direction. However, there was no significant difference in the mucosal apoptosis between the two groups (p=0.892) (Fig. 3E, F). TNF-α, MMP-2 and MMP-9 were expressed, especially on the periphery of the cyst walls or in granulation tissues (Fig. 4). TNF-α, MMP-2 and MMP-9 were found in 38%, 62% and 100% of the PG group, and in 33%, 78% and 89% of the NG group, respectively. There were no significant differences in the percent of TNF-α, MMP-2 and MMP-9 between the two groups (TNF-α: p=0.858; MMP-2: p=0.490; MMP-9: p=0.331).
The pathogenesis of mucoceles, has been described as due to the destruction of the excretory ducts of salivary glands, due to traumatic cutting or obstruction, inducing extravasation of sialomucin into the submucosa, which subsequently produces inflammation2,4,6,7. However, the complete explanation of the pathogenesis of mucoceles remains to be elucidated. Thus, we attempted to clarify the underlying pathogenesis by differentiating lesions based on their gross appearance, histological features and Immunohistochemical staining results.
We divided oral mucoceles into two groups. As expected, the gross size was larger in the NG group compared to the PG group. On histological examination, the A depth was significantly deeper in the NG group. These results show that the NG mucoceles were usually located deep in the submucosa while the PG mucoceles had a tendency to be located directly below the mucosa. The different depth of the lesions may affect the transparency and color of the lesions. Thus, the PG lesions may look whitish because they are filled with mucus and have a shallow depth. We observed that various inflammatory cell infiltrates were prominent around the mucocele in the PG group, while granulation tissue formation was more prominent in the NG group. The walls of the mucoceles were composed of either a single layer of epithelium or a thin layer of granulation tissue in the PG group, while they were composed of relatively thick layers of granulation tissue in the NG group. These structural differences may affect the clinical appearance of different mucoceles.
In order to explain the histological differences, we evaluated the immunological background of the lesions. Recent studies have demonstrated that stimulated keratinocytes can secrete neuropeptides, such as substance P (SP), and pro-inflammatory cytokines like IL-1α, IL-1β, IL-6 and TNF-α8,9. In addition, extravasated mucus can induce extensive macrophage reactions10. Activated monocytes have been shown to synthesize a transmembrane form of TNF at the site of inflammation and locally release a TNF secretory component11. Thus, TNF-α secreted by keratinocytes and activated monocytes stimulate fibroblast proliferation and induce the synthesis of matrix metalloproteinases (MMPs)6,11-14. Furthermore, TNF-α initiates the recruitment of inflammatory cells13. Based on previous reports, we can infer a temporal series of steps during the pathogenesis of a mucocele.
Trauma to the lip would stimulate mucosal keratinocytes. The stimulated keratinocytes would then secrete pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-α. For cases with deep trauma, traumatic rupture of a salivary duct would result in mucus spilling into the dermis. This mucus would then activate dermal macrophages that would then excrete more TNF-α. Excreted cytokines would induce neutrophil recruitment and subsequent inflammatory responses. They would also stimulate dermal fibroblasts to produce MMP-2 and MMP-9. After these steps, dynamic remodeling of the dermal matrix would occur.
To determine if these cytokines were expressed in the lesions, we examined TNF-α, MMP-2, MMP-9 and the inflammatory infiltrates. The results were as follows: TNF-α was expressed in 33~38% of the lesions, MMP-2 in 62~78% and MMP-9 in 89~100%. However, there were no differences in the expression of TNF-α, MMP-2 or MMP-9 between the PG and NG groups. For CD4 and CD8 immunohistochemical staining, positive T lymphocyte infiltration was observed in both groups, but both CD4(+) and CD8(+) T lymphocytes were more abundant in the PG group.
We also examined mucosal apoptosis. The mucosal apoptosis was most prominent in the basal layer of the oral mucosa, and it gradually decreased in the upward direction. Normally, proliferative activity is most prominent in the basal layer of the epithelium. However, our findings were contrary to this concept. We assume that the excretion of inflammatory cytokines, including TNF-α, during the dynamic remodeling process was the reason for this. Adjacent locations of cytokine sources might have more influence on the basal layer in a paracrine pattern. However, there were no differences in the degree of apoptosis between the two clinical groups.
In conclusion, our results were consistent with previous reports regarding the underlying pathogenesis of oral mucoceles. Although primarily a histological review, we found that the structural differences contributed to the gross morphological appearance of the lesions. In our study, the lesions were larger and deeper in the NG group compared to the PG group. There were also significant differences in the type of infiltrating T lymphocytes and granulation tissue formation between the two groups. These findings suggest that the clinical manifestations of mucoceles may be determined by the type of inflammatory response rather than the matrix remodeling process.
Figures and Tables
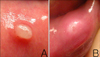 | Fig. 1(A) Asymptomatic, solitary, protruding, horn-shaped, whitish papule on the lower lip. (B) Asymptomatic, solitary, swollen, dome-shaped, erythematous nodule on the lower lip. |
 | Fig. 2(A) A skin biopsy specimen obtained from the lower lip showed a well-defined mucous retention cyst with complete definitive epithelial lining in the lamina propria. (B) A skin biopsy specimen from the lower lip showed intermixing of mucus and granulation tissue in the submucosa. (C) Inside a well-defined cavity, faintly eosinophilic amorphous material, vacuolated macrophages, lymphocytes, and a few eosinophils were observed (A, B: H&E stain, ×40, C: H&E stain, ×400). |
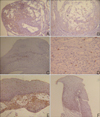 | Fig. 3(A) Many CD4+ lymphocytes were observed in the granulation tissue of a mucocele. (B) Moderate number of CD4+ lymphocytes were observed in the wall and cavity of a mucocele. (C) Moderate numbers of CD8+ lymphocytes were observed in the mucosal basal layer and lamina propria. (D) A few CD8+ lymphocytes were observed in the granulation tissue, which formed the wall of a mucocele. (E) Apoptotic mucosal keratinocytes were observed evenly in the whole layer of the mucosa. (F) Apoptotic mucosal keratinocytes were observed especially on the basal layer of the mucosa (Left column photographs were taken from the PG and right column photographs were taken from NG. A, B: CD4, PAP, ×100, C: CD8, PAP, ×100, D: CD8, PAP, ×400, E, F: apoptosis, PAP, ×100). |
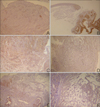 | Fig. 4MMP-2+ materials (A, B), MMP-9+ materials (C, D), and TNF-α+ materials (E, F) were observed in the intercellular spaces of the granulation tissues and cavities of the mucoceles (Left column photographs were taken from PG and right column photographs were taken from the NG. A: MMP-2, PAP, ×100, B: MMP-2, PAP, ×40, C, D: MMP-9, PAP, ×100, E: TNF-α, PAP, ×40, F: TNF-α, PAP, ×200) |
References
1. Ship JA, Phelan J, Kerr AR. Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Biology and pathology of the oral mucosa. Fitzpatrick's dermatology in general medicine. 2003. 6th ed. New York: McGraw-Hill;1087–1088.
2. Lattanand A, Johnson WC, Graham JH. Mucous cyst (mucocele). A clinicopathologic and histochemical study. Arch Dermatol. 1970. 101:673–678.

3. Stetler-Stevenson WG. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996. 148:1345–1350.
4. Chaudhry AP, Reynolds DH, Lachapelle CF, Vickers RA. A clinical and experimental study of mucocele (retention cyst). J Dent Res. 1960. 39:1253–1262.

5. Woo SB. McKee PH, Calonje E, Granter SR, editors. Diseases of the oral mucosa. Pathology of the skin: with clinical correlations. 2005. 3rd ed. Philadelphia: Elsevier Mosby;447.
6. Hoque MO, Azuma M, Sato M. Significant correlation between matrix metalloproteinase activity and tumor necrosis factor-alpha in salivary extravasation mucoceles. J Oral Pathol Med. 1998. 27:30–33.

7. Eversole LR, Sabes WR. Minor salivary gland duct changes due to obstruction. Arch Otolaryngol. 1971. 94:19–24.

8. Dallos A, Kiss M, Polyanka H, Dobozy A, Kemeny L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides. 2006. 40:251–263.

9. Clark JD, Shi X, Li X, Qiao Y, Liang D, Angst MS, et al. Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol Pain. 2007. 3:28.

10. Harrison JD, Garrett JR. Experimental salivary mucoceles in cat. A histochemical study. J Oral Pathol. 1975. 4:297–306.

11. Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990. 63:251–258.

12. Mauviel A. Cytokine regulation of metalloproteinase gene expression. J Cell Biochem. 1993. 53:288–295.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download