Abstract
Acute generalized exanthematous pustulosis is a skin reaction characterized by an acute onset, fever, and a cutaneous eruption with non-follicular sterile pustules on edematous erythema. It mimics many of the features of pustular psoriasis but is differentiated by its characteristic clinical course and history. The cause is usually ingested drugs. We report a case of acute generalized exanthematous pustulosis possibly induced by ritodrine.
Acute generalized exanthematous pustulosis (AGEP) is a cutaneous reaction characterized by widespread tiny sterile pustules induced by a variety of triggering factors, most commonly ingested drugs. The course of the disease is usually very short, less than two weeks in most cases. Skin lesions become generalized within a few days after initiation of the skin reaction and subside quickly with conservative treatment. Frequently, there are also systemic symptoms such as fever and leukocytosis1.
Ritodrine is a widely used tocolytic that relaxes the beta-2-adrenergic receptors in uterine muscles. Skin reactions have been rarely reported with this drug, but it is associated with myocardial infarctions2. Here we report a case of AGEP apparently triggered by ritodrine exposure.
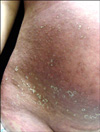
A 26-year-old woman at 32 weeks gestation presented to our department with generalized pruritic pustules and a broad erythematous patch. Ten days previously, she was hospitalized at the local obstetrics department for preterm labor. During her four-day admission, intravenous ritodrine was the only drug used. The patient was then discharged on oral ritodrine. One day after discharge, the patient developed common cold symptoms. The following day, a broad erythematous patch and multiple tiny pustules, accompanied by pruritis, appeared on the trunk (Fig. 1). There was no previous history of any skin disease or allergic reactions to drugs or other materials. The family history was unremarkable.
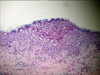
A skin biopsy was performed on a pustule on the abdomen. The findings revealed subcorneal pustules composed of neutrophils with a few necrotic keratinocytes and vacuolization changes (Fig. 2). Laboratory testing showed no abnormalities in the blood counts, blood chemistry, liver function, or renal function except for a slightly increased neutrophil count (90%). After the diagnosis of AGEP was confirmed, the patient was treated with wet saline compresses only. The skin lesions subsided in six days, and one week later she gave birth to healthy male twins.
Five months later, the patient returned to our department and a patch test was performed to determine the cause of AGEP. The powder from the capsule of ritodrine (LAVOPA SR®, Choong Wae Pharm, Seoul, Korea) was separated from the gel jacket and diluted to 10% and 30% in white petrolatum. The gel jacket was moistened with saline before being placed in the patch test chamber. The patch test was performed using white petrolatum and saline as the control. The patch was removed after one day and the results were assessed on day 2 and day 7, with no noticeable reaction.
AGEP is a skin reaction induced by drugs or infection that is characterized by generalized erythroderma, tiny sterile pustules, and a rapid clinical course3. AGEP is often considered a subtype of pustular psoriasis, but Beylot et al.1 reported it as a separate entity. Unlike pustular psoriasis, AGEP patients had no history of psoriasis, no psoriatic changes on the histopathology, had a short clinical course, and the skin lesions were mostly distributed on the flexor surfaces4.
Ritodrine is a well-known β-antagonist used to stop preterm labor. The common adverse effects include: blurred vision, chest pain, headache, drowsiness, mouth dryness, and palpitations2. However, there have been no reports of cutaneous side effects besides dry skin. An initial diagnosis of impetigo herpetiformis was initially made due to the pregnancy, but was later ruled out after reviewing the history of skin disease and histological findings. In addition, the clinical course was about 10 days, which is very short. All of the skin lesions subsided a week before delivery. The bacterial cultures from the pustules resulted in no pathogenic growth and the laboratory tests showed no sign of infection. The patient was admitted to our obstetrics division for observation of a high risk pregnancy; during this time the evolution of the skin lesions was closely monitored. The lesions appeared as tiny pustules from the erythrodermic patch and then disappeared, leaving the characteristic flaking of the scales that are consistent with AGEP. The biopsy findings showed subcorneal pustules without epidermal hypertrophy or acanthosis, which also confirmed the final diagnosis of AGEP.
Various agents can trigger AGEP. According to the Euro-SCAR-study reported by A. Sidoroff et al.5, drugs commonly associated with AGEP include: pristinamycin, aminopenicillins, quinolones, (Hydroxy) chloroquine, sulphonamide, terbinafine, and diltiazem. Other agents such as corticosteroids, macrolides, oxicam NSAIDs, and anticonvulsants, except for valproic acid, showed weak possibilities.
To identify the causative drug, we performed a patch test. Patch testing is one of the most common methods for determining the causative agents of cutaneous drug reactions, and has about 50% sensitivity for AGEP6. Application of the patches is recommended at concentrations between 10% and 30%, as false negative and positive results are more common at other concentrations7. The patient declined intradermal injection and the prick test.
Despite the negative results of the patch test, ritodrine was probably the agent responsible for the skin rash based on a review of the medical history. The patient was pregnant for more than 30 weeks, which reduced the likelihood of ingestion of other drugs. Therefore, this case likely illustrates a rare cutaneous reaction to ritodrine manifesting as AGEP, the first such case to be reported in the English literature.
Figures and Tables
References
1. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases). Ann Dermatol Venereol. 1980. 107:37–48.
2. Yaju Y, Nakayama T. Effectiveness and safety of ritodrine hydrochloride for the treatment of preterm labour: a systematic review. Pharmacoepidemiol Drug Saf. 2006. 15:813–822.

3. Beylot C, Doutre MS, Beylot-Barry M. Acute generalized exanthematous pustulosis. Semin Cutan Med Surg. 1996. 15:244–249.

4. Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001. 28:113–119.

5. Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007. 157:989–996.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download