Abstract
Argyria is a rare cutaneous discoloration caused by the intake of silver or various compounds containing silver. We report a case of argyria in a 73-year-old male following ingestion of colloidal silver as an alternative medicine over 5 years. He had a diffuse, slate gray discoloration of his face and hands. A biopsy specimen from the face revealed brown-black extracellular granules in the upper dermis and between collagen bundles. We also found silver particles in the mucous of the colon. The ingestion of colloidal silver appears to be increasing among patients using alternative health practices. We report this case to bring people's attention to the problems associated with the ingestion of colloidal silver.
Argyria is a cutaneous discoloration caused by the intake of silver or various substances containing silver. Because silver is not absorbed through normal skin, it is assumed that argyria is caused by the absorption of silver via ingestion or implantation from medical instruments. For example, there have been some cases reported of argyria caused by silver used in medical applications1,2. In addition, there have been reports of argyria being induced by colloidal silver as a food supplement3,4. Here, we report a case of argyria in a 73-year-old male following ingestion of colloidal silver as a form of alternative medicine.
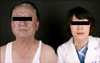
A 73-year-old male visited our clinic with a 2 year history of slate gray colored pigmentation of the face. One year previously, he had visited our clinic with bluish gray discoloration of his entire face. At that time, we recommended further evaluation for his skin lesion, but he refused and stopped visiting our clinic. He later returned because the discoloration began getting lighter and spreading.
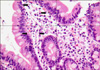
Physical examination revealed bronze-colored pigmentation on his face (Fig. 1). Pigment freckles were not seen on the lips or mouth, and the non-exposed parts of the limbs and trunk were normal. He was currently taking regular medication for hypertension, diabetes and benign prostate hypertrophy; however, none of these medications had been changed over the last few years. Further questioning regarding the use of alternative medicines revealed that the patient had ingested colloidal silver on occasion for at least the last 5 years. We estimated that the mean amount of daily ingested colloidal silver to achieve a concentration of 0.15µg/ml is 700 ml. This gives one an idea that the total amount of ingested colloidal silver for the last 5 years comes to 0.2 g. We suspected pigmented allergic contact dermatitis and metal-induced hyperpigmentation. Therefore, we performed a biopsy of the skin lesion. Microscopic evaluation of a skin biopsy of the forehead at low power revealed upper dermal mild perivascular lymphocyte infiltration. High powered observation of the same sample revealed brown-black extracellular granules in the upper dermis and between collagen bundles (Fig. 2). The patient had undergone colonoscopy and a biopsy of the colonic mucous before visiting our clinic. Silver particles were observed in the mucous of the colon (Fig. 3). We then evaluated the level of silver in the patient's urine, blood, skin tissue and ingested water and found that the silver level of all specimens collected was elevated when compared with reference values (Table 1).
Based on these results, this case was diagnosed as argyria. We advised the patient to discontinue the ingestion of silver and encouraged him to use sunscreen and sun protection to help prevent further skin discoloration. After adopting these practices, the patient's condition improved rapidly.
The ingestion of colloidal silver appears to be increasing among patients using alternative health practices; however, this practice can lead to specific pathological and clinical abnormalities. Many websites identify colloidal silver as a drug that can be used for the treatment of major illnesses such as AIDS, cancer, and arthritis. The patient described in this study had ingested colloidal silver to treat hypertension and diabetes. When he purchased the colloidal silver, he was not warned about its potential side-effects.
As observed in the present case, argyria pigmentation is usually only recognized in sun-exposed areas. Even though the silver is deposited in both exposed and unexposed areas of the skin, the discoloration is more pronounced in areas that have been exposed to sunlight, such as the face and hands. It has been suggested that the deposited silver stimulates melanocytes to produce more melanin, and that this stimulation is increased in the presence of sun exposure. Alternatively, the colorless silver in the dermis becomes brown-black in response to a reduction reaction that occurs when exposed to sunlight3,5.
Although argyria results in skin discoloration, it is generally a benign condition6,7. However, there have been a few reported cases of negative effects on night vision8, as well as isolated reports of neurological deficits, renal problems, and hepatic complications associated with argyria and silver poisoning2.
Based on animal and human experiments, up to 10 percent of silver that is ingested is absorbed in the small intestine9. However, this percentage may be much higher if the mucous membranes of the oral cavity are disrupted. Following ingestion, the highest concentrations of silver are found in the skin, liver, spleen and adrenals. Although it was originally believed that silver does not penetrate the blood-brain barrier, it has since been shown that parenterally administered silver salts can accumulate in neurons and glial cells of the brain and spinal cord10.
Typical histological findings include brown-black granules, singly or in clusters, in the basement membrane zone surrounding the sweat glands and connective tissue sheaths around the pilosebaceous structures11. Examination of an unstained biopsy section by darkfield illumination demonstrates the presence of silver granules outlining the basement membrane of the epidermis and the eccrine sweat glands12. Electron probe microanalysis can be used to confirm the diagnosis. In the present study, we observed silver particles on the skin and colonic mucous biopsy specimens. In addition, we were able to make a quantitative determination of the silver content in the urine, blood, and ingested water using atomic absorption spectrometry (SpectrAA 280Z, Varian Medical System, Inc, USA).
The differential diagnosis for blue-gray discoloration of the skin includes exposure to other heavy metals besides silver, such as mercury, bismuth, arsenic, and gold3. Mercury-induced pigmentation involves skin folds and is not photodistributed and histologic examination of lesions shows large brown-black granules in the papillary dermis around capillaries as well as elastic and collagen fibers13,14. Bismuth hyperpigmentation generally presents as a blue-black line at the gingival margin, although generalized pigmentation may occur13. Arsenic hyperpigmentation occurs as a result of increased melanin synthesis with excess pigment being present at all levels of the epidermis15. Gold hyperpigmentation (chrysiasis) is characterized by deposits of small black macrophage-bound particles surrounding the vessels in the deeper reticular dermis and around the sweat gland coils16. Other causes to be considered include central cyanosis, methemoglobinema, melanosis secondary to widespread melanoma, hemochromatosis, Addison's disease, chlorpromazine, amiodarone, and antimalarial therapy3. Histologically, chlorpromazine hyperpigmentation is characterized by the presence of golden-brown macrophage-bound granules surrounding the superficial vasculature17 and amiodarone pigmentation is characterized by the presence of macrophages containing PAS-positive, yellow-brown lipofuscinlike granules predominantly located in a perivascular distribution18.
Treatment of cutaneous involvement is difficult. The use of depigmentation creams has been ineffective, and chelation attempts are also typically unsuccessful. However, sunscreens and cosmetics can be beneficial for preventing further depigmentary changes and masking discoloration. Although the pigmentary changes in response to argyria are usually permanent1, the subject evaluated here showed some improvement after discontinuing use of the product and staying away from sunlight. Furthermore, no aggravation of the condition was observed after ceasing the use of colloidal silver.
Here, we describe a case of argyria following the ingestion of colloidal silver to bring people's attention to the problems associated with the ingestion of colloidal silver.
Figures and Tables
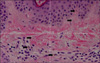 | Fig. 2Brown-black extracellular granules (closed arrows) were found in the upper dermis and between collagen bundles (H&E, ×400). |
References
1. Fung MC, Bowen DL. Silver products for medical indications: risk-benefit assessment. J Toxicol Clin Toxicol. 1996. 34:119–126.

2. Payne CM, Bladin C, Colchester AC, Bland J, Lapworth R, Lane D. Argyria from excessive use of topical silver sulphadiazine. Lancet. 1992. 340:126.

3. White JM, Powell AM, Brady K, Russell-Jones R. Severe generalized argyria secondary to ingestion of colloidal silver protein. Clin Exp Dermatol. 2003. 28:254–256.

4. Park JW, Jung KE, Jin WW, Jung JG, Ro KW, Kim MH, et al. A case of generalized argyria caused by ingestion of silver solution. Korean J Dermatol. 2007. 45:1087–1089.
5. Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal "photograph," a manifestation of passive photo-sensitivity. J Am Acad Dermatol. 1987. 16:211–217.

6. Hill WR, Montgomery H. Argyria: with special reference to the cutaneous histopathology. Arch Derm Syphilol. 1941. 44:588–599.
7. Tanner LS, Gross DJ. Generalized argyria. Cutis. 1990. 45:237–239.
8. Rosenman KD, Moss A, Kon S. Argyria: clinical implications of exposure to silver nitrate and silver oxide. J Occup Med. 1979. 21:430–435.
9. Hill WR, Pillsbury DM. Argyria; the pharmacology of silver. 1939. Baltimore: The Williams & Wilkins Company;128–132.
10. Wadhera A, Fung M. Systemic argyria associated with ingestion of colloidal silver. Dermatol Online J. 2005. 11:12.

12. Peterson WC Jr. Argyria. Minn Med. 1968. 51:533–534.
13. Granstein RD, Sober AJ. Drug- and heavy metal--induced hyperpigmentation. J Am Acad Dermatol. 1981. 5:1–18.

14. Boyd AS, Seger D, Vannucci S, Langley M, Abraham JL, King LE Jr. Mercury exposure and cutaneous disease. J Am Acad Dermatol. 2000. 43:81–90.

16. Smith RW, Leppard B, Barnett NL, Millward-Sadler GH, McCrae F, Cawley MI. Chrysiasis revisited: a clinical and pathological study. Br J Dermatol. 1995. 133:671–678.

17. Wolf ME, Richer S, Berk MA, Mosnaim AD. Cutaneous and ocular changes associated with the use of chlorpromazine. Int J Clin Pharmacol Ther Toxicol. 1993. 31:365–367.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download