Abstract
Kaposi sarcoma (KS) is a multifocal vascular neoplasm most commonly seen in association with human immunodeficiency virus infection. However, KS has also been reported in patients treated with immunosuppressive agents. However, it is very rare to find KS in association with idiopathic thrombocytopenic purpura (ITP). Here we describe a 58-year-old male patient suffering from ITP treated with corticosteroid therapy who developed KS and present a review of the literature.
An increased incidence of Kaposi sarcoma (KS) has been described in organ transplant recipients receiving immunosuppressive therapy1. In addition, KS has been reported in patients treated with corticosteroid therapy for several different clinical entities2-4. However, it is very rare to find KS in association with idiopathic thrombocytopenic purpura (ITP). We now report a case of KS that developed during corticosteroid therapy for ITP.
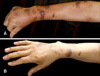
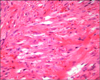
In April 2008, a 58-year-old man presented at a local clinic because of bleeding of the gums. Physical examination revealed several purpuric patches on his legs and no splenomegaly. On laboratory examination, the platelet count was 3×103/ul and anti-platelet antibodies were weakly positive. But anti-HIV antibodies were negative. Therefore, the patient was diagnosed with ITP. He was treated with intravenous human immunoglobulin (9 g/day for 5 days) followed by oral prednisolone (80 mg/day for 1 month). After one month, the platelet count increased to 16.3×103/ul. However, multiple, purplish to black-colored nodules had developed on his extremities and trunk (Fig. 1A). In June 2008, the patient was referred to our clinic and a skin biopsy was performed on his wrist. Histopathology showed proliferation of spindle cells that were arranged in bundles with irregular and slit-like vascular spaces (Fig. 2). Immunohistochemical staining for CD34 and P53 was positive in the endothelial cells and some of the spindle-shaped cells. But there was no visceral involvement on abdominal CT examination. The lesion was diagnosed as KS. Because the immunosuppression induced by corticosteroid therapy was thought to be the cause of the KS, corticosteroid was tapered to 10 mg/day and administration of etoposide (50 mg/day) was initiated. The patient was treated with six cycles of etoposide. After 6 months, the skin nodules had decreased in size and number (Fig. 1B). He was instructed to return to the hospital regularly thereafter.
Four types of KS are recognized: (i) Classic KS; (ii) African endemic KS; (iii) AIDS-associated KS; (iv) KS in therapeutically immunosuppressed patients. Iatrogenic KS was first described among organ transplant recipients1. KS has been reported less frequently during immunosuppressive therapy for a variety of clinical disorders such as rheumatologic disease, hematologic disease and pulmonary disease. More recently, corticosteroid therapy has been associated with the development of KS5. Therefore, corticosteroid induced immunosuppression appears to constitutes a risk factor for the development of KS.
The mechanism of corticosteroid action involves passive diffusion through the cell membrane, movement to the nucleus and regulation of the transcription of target genes6. Systemic corticosteroids are potent immunosuppressive and anti-inflammatory agents. Recently, it was demonstrated that there is a strong inhibition of apoptosis by glucocorticoids at the transcriptional level at all stages of fibrosarcoma development. The regulation of tumor cell death by corticosteroids was shown in vitro during tumorgenesis7.
When we made a diagnosis of both KS and ITP was made in an HIV-negative patient, there were three possible explanations. First, KS might have induced the ITP by vessel wall abnormalities and abnormal sequestration of platelets in peripheral tissues8. Second, patients with ITP are usually treated with immunosuppressive agents such as corticosteroids. As a result, KS might have developed secondary to his immunosuppressed status. Third, KS might have independently occurred along with the ITP by chance or by some unknown mechanisms. Our case was most likely explained by the second mechanism. Indeed, we found three reported cases similar to our case (Table 1)9-11.
The interval between the initiation of steroid therapy and the development of KS has been reported as 3 to 36 months, the average being 13.7 months5. In our case, the interval was two months, which was shorter than that previously reported. In prior cases with a short interval, the underlying disease included disorders with unique susceptibility to the development of KS such as chronic lymphatic leukemia or autoimmune disease. However, our patient had no history of these diseases. On the other hand, the patient had ITP, which might have contributed to the development of KS. Therefore, patients with ITP might be at risk for developing KS. However, an underlying mechanism for this is not known.
The prognosis of corticosteroid-induced KS is unpredictable. In about half of the cases, cessation of the corticosteroid is followed by resolution of the KS, whereas in the other half, the KS progresses5. These variable outcomes suggest that steroids are responsible for induction of precipitating factors for KS. In conclusion, it should be kept in mind that KS can develop during immunosuppressive treatment, even during corticosteroid therapy for ITP.
Figures and Tables
Fig. 1
(A) Multiple, dark-red colored nodules on the extremities that were several centimeters in size. (B) After the cessation of corticosteroids for 6 months, the skin nodules decreased in sized and number.
References
1. Penn I. Kaposi's sarcoma in organ transplant recipients: report of 20 cases. Transplantation. 1979. 27:8–11.

2. Vincent T, Moss K, Colaco B, Venables PJ. Kaposi's sarcoma in two patients following low-dose corticosteroid treatment for rheumatological disease. Rheumatology (Oxford). 2000. 39:1294–1296.

3. Casoli P, Tumiati B. Rheumatoid arthritis, corticosteroid therapy and Kaposi's sarcoma: a coincidence? A case and review of literature. Clin Rheumatol. 1992. 11:432–435.

4. Casoli P, Tumiati B. Kaposi's sarcoma, rheumatoid arthritis and immunosuppressive and/or corticosteroid therapy. J Rheumatol. 1992. 19:1316–1317.
5. Trattner A, Hodak E, David M, Sandbank M. The appearance of Kaposi sarcoma during corticosteroid therapy. Cancer. 1993. 72:1779–1783.

6. Bloom E, Matulich DT, Lan NC, Higgins SJ, Simons SS, Baxter JD. Nuclear binding of glucocorticoid receptors: relations between cytosol binding, activation and the biological response. J Steroid Biochem. 1980. 12:175–184.

7. Gascoyne DM, Kypta RM, Vivanco MM. Glucocorticoids inhibit apoptosis during fibrosarcoma development by transcriptionally activating Bcl-xL. J Biol Chem. 2003. 278:18022–18029.

8. Wijermans PW, van Groningen K, van Royen EA, Bruijn JA. Kaposi's sarcoma in an HIV-negative CLL patient as the cause of thrombocytopenia. Ann Hematol. 1994. 68:307–310.

9. Turnbull A, Almeyda J. Idiopathic thrombocytopenic purpura and Kaposi's sarcoma. Proc R Soc Med. 1970. 63:603–605.

10. Kohno M, Miyata M, Ohmoto A, Matsuyama R. Kaposi's sarcoma developing during corticosteroid therapy in idiopathic thrombocytopenic purpura. Rinsho Ketsueki. 1989. 30:1310–1313.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download