Abstract
Background
Several different kinds of drugs have been used to treat chronic oral lichen planus (OLP). During the last decade, there have been several reports demonstrating success with levamisole and low dose prednisolone therapy for treating OLP. However, some OLP patients who have underlying diseases such as diabetes, hypertension and malignancy are unable to take steroids.
Methods
Eleven patients who had OLP were treated with levamisole between 2005 and 2007. The levamisole was administered at a dose 50 mg thrice daily for three consecutive days, but then it was not administered on the following four days.
Results
After 2 weeks of treatment, 8 patients reported a partial response, 3 patients reported no response and no patients reported clearance of lesion. After 4 weeks of treatment, 6 patients reported a partial response, 3 patients reported no response and 2 patients reported clearance of lesion. Furthermore, after 3 months of treatment, 3 patients reported a partial response, 3 patients reported no response and 5 patients reported complete clearance of lesion. Clinical improvement was shown in 2 weeks, whilst the mean duration to achieve clearance of lesion was 6.2 weeks. Although 1 patient had mild itching, there were no significant adverse effects.
Oral lichen planus (OLP) is defined as a T-cell mediated inflammatory disease of the oral mucosa1. OLP is a chronic disease and it rarely undergo spontaneous remission, and it has the potential to become malignant2. The conventional treatments for OLP are topical and systemic corticosteroids, mycophenolate mofetil, cyclosporine, methotrexate, dapsone, griseofulvin, retinoids, pimecrolimus, tacrolimus, PUVA, 308 nm-excimer laser, hydroxychloroquine and low molecular weight heparin (enoxaparin)3-13. Most patients have been treated with steroids, yet many patients who have underlying diseases such as diabetes, hypertension and malignancy have limitations for using immunosuppressants.
During the last decade, there have been several reports demonstrating the success with using levamisole and low dose prednisolone therapy for oral lichen planus14-16. Indeed, the patients with OLP showed a dramatic response to this therapy14,15. Levamisole is an effective immunomodulating agent that can restore the normal phagocytic activity of macrophages and neutrophils, it modulates T-cell mediated immunity and it potentiates the activity of human interferon17-21. It is used to treat several malignancies and autoimmune diseases (for example, rheumatoid arthritis and systemic lupus erythematosus), vitiligo and viral warts22-29.
In this article, we present a case series for evaluating levamisole monotherapy without steroids for treating OLP.
We performed a retrospective medical chart review of 11 patients who were diagnosed with OLP and we subsequently treated these patients with levamisole monotherapy at the Dermatology Department of Wonkwang University Hospital from 2005 to 2007. This study was approved by the Institutional Review Board at Wonkwang University Hospital, Iksan, Korea. The diagnosis of OLP was made by the clinical history, the physical examination and the histopathologic findings. Any patients who had an OLP-like lesion induced by drugs or dental prosthetics were excluded from the study. All the patients had drug washout periods of 3 months. On the retrospective chart review, we obtained the following information: the disease site, the disease duration, the previous treatment, the subjective and objective clinical responses to levamisole monotherapy, the adverse effects during levamisole monotherapy and the skin biopsy findings. Before the initiation of levamisole monotherapy, we performed a baseline complete blood cell count, liver and kidney function tests and chest x-ray. A blood test was performed once a month and a skin biopsy was performed in the event of a reticulated or ulcerated lesion.
The levamisole was administered at a dose of 50 mg thrice daily for three consecutive days, and then it was not administered on the following four days. This treatment cycle was repeated until the OLP oral lesion was cleaned out. Every patient was evaluated every 2 weeks, and they were evaluated again at least 3 months.
Based on objective clinical improvement, which was retrospectively analyzed by the patients' charts and the clinical photos, the patients were categorized as having achieved complete clearance, a partial response or no changes. One hundred percent remission of the patch and erosion was defined as complete clearance. A partial response was defined when some lesions of the patch or the erosion were remitted on physician's gross examination. No improvement or worsening of the disease was defined as no change. Treatment failure was classified as those patients who had no clinical improvement at least once during treatment over a 3 month period. The lesions that recurred after complete or partial improvement were counted as a complete or partial response, and we recorded the disease free-duration and the number of recurrences.
Eleven patients were diagnosed as having OLP and they were treated with levamisole monotherapy. Eight patients (72.7%) were female and the mean age of all the patients was 42.6 years (range: 20~61 years). The mean duration of OLP was 20.8 months (range: 3 months~5 years). All the patients had OLP on the buccal mucosa, except patient #1 who had OLP on the tongue base. All the patients had a whitish reticulated patch, except 1 patient who had an ulcerated patch. Three patients had dental prosthetics, but these dental prosthetics were not the cause of the OLP because of the distance between the oral lesion and the dental prosthetics (Table 1).
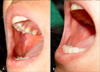
After 2 weeks of treatment, 8 patients (55%) showed a partial response (Fig. 1) and 3 patients had no response. No patient had complete clearance of lesion. Patient #4 had mild itching after receiving the medication, but the itching was controlled with antihistamine medication (Table 2).
After 4 weeks of treatment, 6 patients (72.7%) showed a partial response, 2 patients (18.2%) had complete clearance and 3 patients again showed no response (Table 2). After 3 months of treatment, 3 patients (72.7%) showed a partial response and 5 patients (45.5%) showed complete clearance. However, 3 patients showed no response, so they were defined as treatment failures. Interestingly, the disease duration of the patients who showed no response was shorter than that of the patients who had a response (Table 2).
Eight patients, except 3 patients who had no response, showed clinical improvement at the next visit (after 2 weeks). Complete clearance occurred in 5 patients and their treatment duration was 3 weeks to 3 months (mean: 6.2 weeks). However, patient #1 had several recurrences. During the 2 year follow up period, patient #1 had a total of 6 recurrences, but the lengths of disease free periods were increased.
No patient had any significant side effects or laboratory changes. Patient #4 had mild itching after receiving medication at week 2, but this resolved quickly with the use of an antihistamine (Table 3).
Oral lichen planus is a chronic and troublesome inflammatory oral disease. Even though there are many treatment options available for OLP, it is very hard to effectively treat this malady.
Levamisole is a levisomer of tetramisole ((-)-2,3,5,6-tetrahydro-6-phenylimidazole [2,1-6] thiazole monohydrochloride), and has been used as a broad spectrum anti-helminthic drug since 1966.
In 1978, Renoux et al.30 reported that levamisole increased cellular immunity. In 1990, the FDA approved levamisole for many autoimmune and inflammatory diseases. Levamisole is currently used for the treatment of vitiligo, viral warts, systemic lupus erythematosus, rheumatoid arthritis and colon cancer22-29.
The first treatment trial of levamisole for OLP was conducted by Lu et al.14 in 1995. In that trial, 23 patients were treated with levamisole (150 mg/d, 3 times per week) and low dose prednisolone. After 2 weeks of treatment, 12 patients showed over 80% improvement, whereas 11 patients showed no response. After 4 weeks of treatment, 23 patients had over 80% improvement and the remission duration was 9.5 months. Because most of the OLP patients in our trial had many underlying diseases, they were restricted from taking steroids, so we tried to treat the OLP without steroids. In our study, after 2 weeks of levamisole monotherapy, 6/11 patients (55%) showed clinical improvement. After 4 weeks of treatment, 2 patients (18.2%) showed complete clearance and 8 patients (73%) showed clinical improvement. After 3 months of treatment, 5 patients (45.4%) showed complete clearance of lesion. However, 3 patients showed no response from the initiation of this therapy.
The treatment effect of levamisole monotherapy might be lower and slower than that of levamisole combined with low dose prednisone. Nevertheless, levamisole monotherapy was shown to be effective for the treatment of OLP. Therefore, levamisole monotherapy can be recommended for those OLP patients who are unable to take steroids.
The number of patients who showed no response was 3. Interestingly, they had a short duration of disease (3, 6 and 6 months, respectively). The mean disease duration of the responders was 26.8 months and they had undergone many treatment trials with no response. At this point, we thought that levamisole monotherapy was a first line drug for treating these chronic OLP patients.
Only 1 patient had an adverse effect, which was itching after receiving medication. This was resolved by using an antihistamine (hydroxyzine Hcl). Levamisole has been reported to have many adverse effects such as nausea, vomiting, fever, dizziness, headache, tiredness, skin rash, anaphylaxis and most severely, agranulocytosis20,21,30-33. Agranulocytosis is commonly seen in those patients with HLA-B27 positivity and in those patients who have undergone long term levamisole therapy32,33. For this reason, levamisole is usually recommended for intermittent use. In rheumatoid arthritis patients, the number of agranulocytosis cases decreased after receiving levamisole 1~2 times a week, and the levamisole showed equivalent efficacy with levamisole continuous therapy34. Therefore, we recommend using levamisole (150 mg/d) 3 times a week with regular blood test monitoring.
In conclusion, we report that levamisole monotherapy is effective for treating OLP patients who are unable to use steroids and who had no response to conventional treatment.
Figures and Tables
References
1. Wilson E. On lichen planus. J Cutan Med Dis Skin. 1869. 3:117–132.
2. Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: a study of 723 patients. J Am Acad Dermatol. 2002. 46:207–214.

4. Eisen D, Griffiths CE, Ellis CN, Nickoloff BJ, Voorhees JJ. Cyclosporin wash for oral lichen planus. Lancet. 1990. 335:535–536.

5. Eisen D, Ellis CN, Duell EA, Griffiths CE, Voorhees JJ. Effect of topical cyclosporine rinse on oral lichen planus. A double-blind analysis. N Engl J Med. 1990. 323:290–294.

6. Eisen D, Ellis CN. Topical cyclosporine for oral mucosal disorders. J Am Acad Dermatol. 1990. 23:1259–1263.

7. Aufdemorte TB, De Villez RL, Gieseker DR. Griseofulvin in the treatment of three cases of oral erosive lichen planus. Oral Surg Oral Med Oral Pathol. 1983. 55:459–462.

8. Massa MC, Rogers RS 3rd. Griseofulvin therapy of lichen planus. Acta Derm Venereol. 1981. 61:547–550.
9. Sehgal VN, Abraham GJ, Malik GB. Griseofulvin therapy in lichen planus. A double-blind controlled trial. Br J Dermatol. 1972. 87:383–385.
10. Kaliakatsou F, Hodgson TA, Lewsey JD, Hegarty AM, Murphy AG, Porter SR. Management of recalcitrant ulcerative oral lichen planus with topical tacrolimus. J Am Acad Dermatol. 2002. 46:35–41.

11. Thomson MA, Hamburger J, Stewart DG, Lewis HM. Treatment of erosive oral lichen planus with topical tacrolimus. J Dermatolog Treat. 2004. 15:308–314.

12. Byrd JA, Davis MD, Bruce AJ, Drage LA, Rogers RS 3rd. Response of oral lichen planus to topical tacrolimus in 37 patients. Arch Dermatol. 2004. 140:1508–1512.

13. Sloberg K, Hersle K, Mobacken H, Thilander H. Topical tretinoin therapy and oral lichen planus. Arch Dermatol. 1979. 115:716–718.

14. Lu SY, Chen WJ, Eng HL. Dramatic response to levamisole and low-dose prednisolone in 23 patients with oral lichen planus: a 6-year prospective follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995. 80:705–709.

15. Lu SY, Chen WJ, Eng HL. Response to levamisole and low-dose prednisolone in 41 patients with chronic oral ulcers: a 3-year open clinical trial and follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 86:438–445.

16. Sun A, Chiang CP, Chiou PS, Wang JT, Liu BY, Wu YC. Immunomodulation by levamisole in patients with recurrent aphthous ulcers or oral lichen planus. J Oral Pathol Med. 1994. 23:172–177.

17. Sun A, Wang JT, Chia JS, Chiang CP. Serum interleukin-8 level is a more sensitive marker than serum interleukin-6 level in monitoring the disease activity of oral lichen planus. Br J Dermatol. 2005. 152:1187–1192.

18. Lewinski UH, Mavligit GM, Hersh EM. Cellular immune modulation after a single high dose of Levamisole in patients with carcinoma. Cancer. 1980. 46:2185–2194.

19. Redondo JM, Lopez-Guerrero JA, Fresno M. Potentiation of interleukin-2 activity by levamisole and imidazole. Immunol Lett. 1987. 14:111–116.

20. Amery WK, Bruynseels JP. Levamisole, the story and the lessons. Int J Immunopharmacol. 1992. 14:481–486.

22. Cho NJ, Choi DY, Son SJ. Therapeutic effect of levamisole in recurrent aphthous stomatitis. Korean J Dermatol. 1979. 17:389–396.
23. Scheithauer W, Kornek GV, Marczell A, Karner J, Salem G, Greiner R, et al. Combined intravenous and intraperitoneal chemotherapy with fluorouracil+leucovorin vs fluorouracil+levamisole for adjuvant therapy of resected colon carcinoma. Br J Cancer. 1998. 77:1349–1354.

24. Sany J. Immunological treatment of rheumatoid arthritis. Clin Exp Rheumatol. 1990. 8:Suppl 5. 81–88.
25. Parsad D, Saini R, Negi KS. Comparison of combination of cimetidine and levamisole with cimetidine alone in the treatment of recalcitrant warts. Australas J Dermatol. 1999. 40:93–95.

26. Amer M, Tosson Z, Soliman A, Selim AG, Salem A, al-Gendy AA. Verrucae treated by levamisole. Int J Dermatol. 1991. 30:738–740.

27. Agarwal S, Ramam M, Sharma VK, Khandpur S, Pal H, Pandey RM. A randomized placebo-controlled double-blind study of levamisole in the treatment of limited and slowly spreading vitiligo. Br J Dermatol. 2005. 153:163–166.

28. Pasricha JS, Khera V. Effect of prolonged treatment with levamisole on vitiligo with limited and slow-spreading disease. Int J Dermatol. 1994. 33:584–587.

29. Rovensky J, Cebecauer L, Zitnan D, Lukac J, Ferencik M. Levamisole treatment of systemic lupus erythematosus. Arthritis Rheum. 1982. 25:470–471.

30. Renoux G, Renoux M, Teller MN, McMahon S, Guillaumin JM. Potentiation of T-cell mediated immunity by levamisole. Clin Exp Immunol. 1976. 25:288–296.
31. Kim HK, Jung YB, Lee EW. Clinical application of D-penicillamine and levamisole in rheumatoid arthritis. J Korean Orthop Assoc. 1983. 18:81–88.

32. Rosenthal M, Trabert U, Muller W. The effect of levamisole on peripheral blood lymphocyte subpopulations in patients with rheumatoid arthritis and ankylosing spondylitis. Clin Exp Immunol. 1976. 25:493–496.
33. Sany J. A review of the effects of levamisole on erythrocyte sedimentation rate, acute phase proteins, and anemia. J Rheumatol Suppl. 1978. 4:43–47.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download