INTRODUCTION
Hydroa vacciniforme (HV) is a rare photosensitivity disorder characterized by recurrent necrotic vesiculopapules on sun-exposed areas, which heal with vacciniform scarring. HV usually starts during childhood and resolves spontaneously during adolescence without systemic involvement.
Recently, there have been reports of patients with atypical HV-like eruptions in Asia and Mexico1-7. In contrast with the symptoms seen in typical HV, these patients showed facial swelling, indurated nodules on non-sun-exposed and sun-exposed areas, high-grade fever, wasting, and hepatosplenomegaly. Several of the reported patients progressed to hematological malignancies and death. Latent Epstein-Barr virus (EBV) infection was detected in most of the patients with this atypical HV-like eruption.
However, Iwatsuki et al8 reported that some patients with atypical HV-like eruptions did not progress to overt malignant hematological neoplasia. In addition, the investigators reported six patients with the typical manifestations of HV who had a number of EBV-encoded small nuclear RNA (EBER)+ cells in the cellular infiltrates in the dermis. These findings support the possibility that typical HV is also associated with latent EBV infection.
CASE REPORT
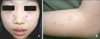
An 11-year-old Korean girl presented with a 5-year history of recurrent, scattered, discrete vesicular eruptions with scars on the face and the extensor surfaces of both forearms. Her skin lesions were aggravated by sun exposure and healed spontaneously with crusts and mild scars. There was no family or personal history of cutaneous photosensitivity. Physical examination showed multiple erythematous papules, vesicles, crusts, and shallow scars on the face, especially on the cheeks (Fig. 1). Except for the skin lesions, there were no remarkable findings on physical examination. Minimal erythemal doses (MEDs) of visible light and UVB were measured with Ektagraphic® (Eastman-Kodak, USA) and UV 800® (Waldmann, Germany) respectively. UVA MED measurements and photoprovocation tests were not performed due to patient rejection. The MED was 50 mJ/cm2 for UVB. No response was observed for a 30-minute exposure to visible light. Initial laboratory investigations showed that the erythrocyte sedimentation rate (27 mm/h) and the antistreptolysin O titer (433, positive) were slightly elevated, and the anti-SSB/La antibody was positive. Other laboratory studies were within normal limits, including complete blood cell count with differential cell count, platelet count, liver and renal function tests, antinuclear antibody concentration, lupus erythematosus cell preparation, anti-SSA/Ro antibody, and anti-Smith antibody.
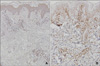
Histopathologic examination showed intraepidermal vesiculation with superficial and deep perivascular lymphocytic infiltrations in the dermis (Fig. 2A). In situ hybridization was carried out on paraffin-embedded skin biopsy sections, using fluorescein-conjugated oligonucleotide probes for EBV early RNAs (EBERs) (Dakopatts, Glostrup, Denmark) for the detection of latent EBV infection. Distinct, dark blue precipitates representing EBER transcripts were found in the nuclei of the lymphoid cells in the dermal infiltrate (Fig. 2B). Immunohistochemical staining was performed using monoclonal antibodies to CD3 (pan T-cell marker), CD45RO (memory T-cell marker), CD8 (helper T-cell marker), CD20 (B-cell marker), CD56 (NK-cell marker) (Dakopatts, Glostrup, Denmark), and latent membrane protein-1 (LMP-1). Most of the infiltrating cells in the dermis had a positive T-cell phenotype: CD3 and CD45RO positivity with some CD8 positivity. Only a few CD56-positive cells were present in the perivascular area. CD20-positive cells and LMP-1-positive cells were absent (Fig. 3).
Based on the clinical and histopathological findings, typical EBV-associated hydroa vacciniforme was suspected. Avoidance of sunlight and sunscreen application was recommended. After 1 year of follow-up, the patient has manifested no recurrence and shows no evidence of lymphoproliferative disorders.
DISCUSSION
Hydroa vacciniforme was first described by Bazin et al13 in 1862. It is clinically characterized by recurrent necrotic vesiculopapules on sun-exposed areas, which heal with vacciniform scarring. It is known to be a disease of early childhood that regresses spontaneously in adolescence and does not impair the general health of the patient14. Iwatsuki et al8 reported 6 patients with clinically and histologically typical HV who had EBV-encoded small nuclear RNA (EBER)+ cells in the dermal infiltrate. They suggested the possibility that typical HV and atypical HV are variants within the same disease spectrum of EBV-associated lymphoproliferative disorders8,15.
However, patients with EBV-associated HV-like eruptions, mainly reported in Asia1-3,16-18, present with atypical skin lesions in non-sun-exposed areas, as well as associated systemic symptoms. They occasionally progress to develop hematological malignancies. Such a clinical entity has been regarded as differing from typical HV, although it has several similar clinical and histopathological findings.
The EBV-associated HV-like eruptions reported in Korea1-3,19 have shown features more typical of EBV-associated lymphoproliferative disorders than of typical HV: skin lesions recurring continuously irrespective of sun exposure, associated systemic symptoms, with most patients progressing to develop hematological malignancies. Thus, in contrast to the preponderance of typical HV cases seen in Caucasians, most of the cases reported in Asians, including Koreans, show EBV-associated HV-like eruptions with malignant potential.
In the current case, clinical manifestations were similar to those seen in typical HV, with a benign course for the skin lesions, which cleared up after photoprotection was implemented. Associated latent EBV infection was confirmed by the presence of EBV-related RNAs, similar to those seen in the patients described by Iwatsuki et al8.
Immunohistochemical staining revealed that the EBER-ontaining cells predominantly had a T-cell phenotype (CD3, CD45RO). Only a few cells had an NK-cell (CD56) phenotype, and no cells had a B-cell (CD20) phenotype. No patients expressed LMP-1, similar to the patients described by Iwatsuki et al8. LMP-1 is a gene product of latent EBV infection and is known to have oncogenic activity18. However, detection of this gene product is not suitable for a screening test for EBV infection, since LMP-1 is not always expressed by EBV-infected cells20.
Previously, our group reported 6 patients with EBV-associated lymphoproliferative lesions presenting as HV-like eruption, with 3 different clinical courses19. The varying clinical courses in patients with EBV-associated HV-like eruptions are considered to be associated with the number of EBER+ cells in the skin lesions, subtype of EBV, and immune status of the patient16. Detailed pathophysiological mechanisms related to the various clinical manifestations occurring in the same disease spectrum remain to be determined.
Six cases of typical HV have been reported in Korea9-12, including a case of HV confirmed by repetitive UVA phototesting that showed spontaneous remission with the use of topical sunscreen9. To our knowledge, there has been no previous report of typical HV with confirmed latent EBV infection in Korea. Hence, we report this interesting case, which we believe to represent the first reported case of this entity in Korea.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download