Abstract
Background
Malassezia yeasts are normal flora of the skin that are discovered in 75~98% of health subjects, but since its association with various skin disorders have been known, many studies have been conducted in the distribution of the yeasts.
Objective
To isolate, identify, and classify Malassezia yeasts from the normal human skin of Koreans by using the rapid and accurate molecular biology method (26S rDNA PCR-RFLP) which overcome the limits of morphological and biochemical methods, and to gather a basic database that will show its relation to various skin diseases.
Methods
Malassezia yeasts were cultured from clinically healthy human skin using scrub-wash technique at five sites (forehead, cheek, chest, upper arm, and thigh) and swabbing technique at scalp in 160 participants comprised of 80 males and 80 females aged from 0 to 80. Identification of obtained strains were placed into the one of the 11 species by 26S rDNA PCR-RFLP.
Results
An overall positive culture rate was 62.4% (599/960). As shown in the experiment groups by their age, the positive culture rate was the highest (74.2%) in the age 21~30 and 31~40 (89/120). In the experiment groups by different body areas, the scalp showed the highest positive culture rate of 90% (144/160). On analysis of 26S rDNA PCR-RFLP, M. globosa was the most predominant species in the age 0~10 (32.8%), 11~20 (28.9%), 21~30 (32.3%). M. restricta was identified as predominant species in the age 41~50 (27.9%), 61~70 (31.5%) and 71~80 (24.0%). In the age 31~40 years, M. sympodialis was found to be the most common species (24.6%). According to body site, M. restricta was more frequently recovered in the scalp (56.8%), forehead (39.8%) and cheek (24.0%) and while M. globosa was more frequently recovered in the chest (36.8%). Higher positive culture rates of Malassezia yeasts were shown in male subjects than female counterparts in all body areas except scalp (p<0.05). Especially in this study, M. dermatis, newly isolated Malassezia species from atopic dermatitis patient in Japan, was isolated and identified in 19 cases (1.9%) in healthy subjects.
Conclusion
The key is to recognize the existence of a difference in the type of Malassezia species in different ages as well as body areas, which reflects differing skin lipid levels in various ages and different body areas. Moreover, 26S rDNA PCR-RFLP analysis which was opted in this study could provide a sensitive and rapid identification system for Malassezia species, which may be applied to epidemiological surveys and clinical practice.
The Malassezia yeasts are lipophilic fungi which are regarded as normal flora of the skin, and are recovered in 75~80% of healthy adults1,2. Malassezia yeasts, since first reported in 1889, are known to be implicated in various diseases, including pityriasis versicolor, seborrheic dermatitis, and Malassezia folliculitis. Recently, there have been a growing number of reports which show the implication of Malassezia yeasts in atopic dermatitis, confluent and reticulated papillomatosis (Gougerot-Carteaud), and Malassezia onychomycosis3-8. Furthermore the pathogenicity of Malassezia yeasts comes into the surface as systemic infections with Malassezia are identified in premature neonates and immunodeficient adult patients undergoing lipid replacement therapy via vein catheter9,10.
It was classified into 7 species, M. furfur, M. pachydermatis, M. sympodialis, M. globosa, M. obtusa, M. restricta, and M. slooffiae in 1996 by Guého et al11 on the basis of molecular biology and by employing interdisciplinary approach of morphology, microstructure analysis, and physiology, and many researches have been done on the yeasts species mainly cultured in Malassezia associated skin diseases. In many researches about Malassezia yeasts, authors have used morphological analysis of the size, surface, color, and shape of cultured colony and biochemical analysis12-15. However, while morphologic analysis is the appropriate method for classification and identification of fungus, it is usually time-consuming, multi-step process necessitating several experimental techniques, does not take taxonomic component into consideration and thus genetic link between species is not possible. So the morphological and biochemical methods have many limits in identification and classification of new species. To overcome such limits of the methods, in the recent researches use various molecular biological methods such as nested polymerase chain reaction (PCR)16, real-time PCR17, pulsed field gel electrophoresis (PFGE)18, amplificated fragment length polymorphism (AFLP)19,20, denaturing gradient gel electrophoresis (DGGE)20, random amplification of polymorphic DNA (RAPD)21-23, single strand conformation polymorphism (SSCP)24, terminal fragment length polymorphism (tFLP)25, restriction fragment length polymorphism (RFLP)24-28, and sequencing analysis29 were used. Recently, on the basis of DNA relatedness through the molecular biology, four new species have been included: M. dermaits, M. japonica, M. nana, and M. yamatoensis29-32. Moreover, there are ongoing researches on Malassezia associated skin diseases such as atopic dermatitis, seborrheic dermatitis, and pityriasis versicolor to analyze the distribution of Malassezia and the subtyping of a specific species through the molecular biological methods. However, there are just a few studies on the qualitative character and distribution of the Malassezia yeasts in various age groups and different body sites in normal human skin using molecular biological techniques.
Therefore, the aim of the present study is to isolate, identify, and classify Malassezia yeasts from the normal human skin of Koreans by using the rapid and accurate molecular biological methods which overcome the limits of morphological and biochemical methods, and is to gather a basic database that will show the relation to various skin diseases. To do so, we classified Malassezia yeasts which have been recovered from the normal skin of Korean subjects using molecular technique and conducted qualitative study. This study was conducted on 160 healthy men and women aged between 0 and 80 years without skin disease. Malassezia yeasts were collected from 6 body sites and were analyzed by 26S rDNA PCR-RFLP which is a more accurate way than any other molecular biological method. And it was also investigated whether the frequency of recovery and distribution of Malassezia yeasts varied with different body areas, sex, and age groups (AGs).
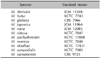
Total 11 standard strains were used in this experiment to obtain the 26S rDNA PCR-RFLP pattern of Malassezia yeasts. Six standard strains were from Korean Collection for Type Cultures (KCTC) of Biological Resource Center; M. furfur (KCTC 7743), M. obtusa (KCTC 7847), M. pachydermatis (KCTC 17008), M. restricta (KCTC 7848), M. slooffiae (KCTC 17431), and M. sympodialis (KCTC 7985). Two standard strains were from Centralbureau voor Schimmelcultures (CBS); M. globosa (CBS 7966) and M. yamatoensis (CBS 9725), and three standard strains were from Japanese Collection of Microorganisms (JCM); M. dermatis (JCM 11348), M. nana (JCM 12085), and M. japonica (CBS 9432) (Table 1).
Specimens were taken from 160 participants comprised of 80 males and 80 females aged from 0 to 80 years, and the subjects were grouped according to their ages (0~10 years: 20 subjects, 11~20: 20 subjects, 21~30: 20 subjects, 31~40: 20 subjects, 41~50: 20 subjects, 51~60: 20 subjects, 60~70: 20 subjects, 70~80: 20 subjects). Six body sites were selected for the examination: scalp, forehead, cheek, chest, upper arm, and thigh. In order to discover the precise distribution pattern of Malassezia yeasts, those who were diagnosed with or treated for the diseases that were presumed to be associated with Malassezia were excluded from the study. All subjects were provided with careful explanation on the purpose and method of the trials and expected results, etc. and all informed consents were received before screening.
Skin sampling by scrub-wash technique was based on the method suggested by Williamson & Kligman33 as a principal method. A stainless steel tube with interior area of 4.909 cm2 was placed onto the skins from the midportion of forehead, cheek, chest, and the medial side of upper arm, thigh, and 1 ml of the prepared detergent (0.01% NaH2PO42H2O, 1.01% Na2HPO4, 0.1% Triton X-100 [pH 7.9]) was dropped through the tube. The skin was then agitated for 1 minute by glass rod, and a specimen was taken, using pipette and was put in a new container. The procedure was repeated in the same way, and the sampled skin was added to the first sample. Scalp samples are difficult to be taken due to hair, thus the swabbing technique was employed instead of scrub-wash technique34. The detailed procedure was as follows: a swab moistened in 100µl of the detergent was rubbed five times against the 3 cm on the scalp skin. Then only the fiber-tipped part of the swab was cut and then placed in 900µl of the detergent. It was placed into a shaker for 30 seconds to make the Malassezia yeasts evenly dispersed in the solution. About 100µl of the sampled skin was mixed with 900µl of the detergent with half of the concentration. From this, 100µl was taken to be applied evenly on the Leeming & Notman culture medium35 and was incubated at 34℃ for 14 days. To isolate cultured Malassezia yeasts, the shape and size of the colony and changes of the media were observed and distinct colonies were collected and analyzed.
For DNA extraction and PCR analysis of both 11 standard strains and isolates from skin, this study adopted colony PCR analysis which was developed to extract DNA directly from colonies of a PCR tube and to amplify 26S rDNA at the same time instead of direct genomic DNA extraction methods. Single colony of Malassezia yeasts was taken and transferred to a PCR tube and warmed up in a double boiler by using a microwave for 1 minute three times, and then the tube was moved into ice water. PCR reaction mixture (0.25 mM deoxynucleoside triphosphate, 10 X PCR buffer, 5 X Q buffer, 0.5µM primers, 1.25 U Hot StarTaq polymerase, 20 mM MgSO4) was added and the vortex mixing was done. Then, PCR using Mastercycler 5333 (Eppendorf, Hamburg, Germany) was immediately performed. To amplify 26S rDNA, the primer that can amplify all 11 standard strains at once was chosen. The sequence was forward, 5'-TAACAAGGATTCCCCTAGTA-3' and reverse, 5'-ATTACGCCAGCATCCTAAG-3'27. The conditions in the early stage of reaction were at 95℃ for 14 minutes for pre-denaturation, at 94℃ for 45 seconds for denaturation, at 55℃ for 45 seconds for annealing, at 72℃ for 1 minute for extension of 40 cycles, and at 72℃ for 7 minutes for last extension. Amplified DNA was visualized by electrophoresis on a 1.5% (w/v) agarose gel with Ethidium bromide (0.5µg/ml) by using 1 X TAE migrating buffer (pH 8.0, 40 mM Tri-acetate 1 mM EDTA).
After the confirmation of the amplified 26S rDNA, PCR products were purified using an Accu-Prep PCR purification kit (Bioneer, Daejeon, Korea). Two restriction enzymes, Hha1 (Takara Biomedicals, Otsu, Japan) and BstF51 (SibEnzyme, Novosibirsk, Russia) were used to analyze 26S rDNA RFLP of Malassezia. In this experiment, the restriction enzyme reaction was performed with 10X PCR buffer, 10 U of the restriction enzyme, and PCR products 7.5µl, which sum up to 20µl. After the reaction at 37℃ for 3 hours, the electrophoresis was done with 3.5% (w/v) NuSieve GTG agarose gel (FMC, Rockland, ME, USA) by 100 volt and stained with ethidium bromide. Restriction fragments were analyzed with the size and number of DNA fragments under the UV transilluminator.
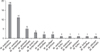
In colony PCR to amplify the 26S rDNA of Malassezia standard strains, a 580 bp PCR band was seen in all of 11 standard strains (Fig. 1). On analysis of PCR-RFLP of 26S rDNA using restriction enzymes Hha1, different restriction patterns were found in nine different species including M. furfur, M. pachydermatis, M. globosa, M. obtusa, M. restricta, M. slooffiae, M. nana, M. japonica, and M. yamatoensis (Fig. 2A) but M. sympodialis and M. dermatis showed the same pattern. So, restriction enzyme BstF51 was additionally used to classify M. sympodialis and M. dermatis. They displayed different patterns and were identified as different species (Fig. 2B). We could differentiate all Malassezia standard strains by analyzing 26S rDNA PCR-RFLP pattern using two restriction enzymes Hha1 and BstF51, and as a result we could gather the database of restriction pattern which was interspecifically distinguishable. As evidenced above, 26S rDNA PCR-RFLP analysis could provide sensitive and rapid identification method for Malassezia species. As the standard strains, we identified 580 bp PCR band from the colony PCR with all of the colonies which were isolated and cultured from the healthy human skin (Data not shown).
The overall positive culture rate of the sampled Malassezia yeasts from the different body sites of total 160 persons was 62.4%, with 599 positive cases out of 960 samples. As shown in this experiment groups by their ages, the positive culture rate was the highest (74.2%) in the age 21~30 and 31~40 recording 89 of 120 cases. The positive culture rate for the age 11~20 was 65.8% (79/120), the age 71~80 was 65.8% (79/120), the age 61~70 was 59.2% (71/120), and the age 51~60 showed the lowest rate of 52.5% (63/120). The results suggest statistically significant difference as illustrated in the success rates by the age groups (p=0.003). Male subjects suggested higher culture rates than female counterparts in all age groups except the age 0~10 (p<0.05). Overall differences among ages and sex were statistically meaningful (p<0.0001) (Table 2).
In the experiment groups by different body areas, the scalp showed the highest positive culture rate of 90% (144/160) (p<0.001). The rate of the forehead and chest was 81.3% (130/160), cheek was 68.8% (110/160), upper arm was 31.9% (51/160), and thigh was 21.3% (34/160). The culture rate of each body site showed statistical significance (p=0.003). Higher positive culture rates of Malassezia yeasts were shown in male subjects than female counterparts in all body areas except scalp (p<0.05) (Table 3).
In many individuals, more than one Malassezia species was recovered from any given body site. Different Malassezia species were recovered among the different colonies picked from the same plate from a given body site. Therefore, this research observed the shape and size of colony and the change of cultured media, and analyzed each species displaying distinct patterns. The results showed that M. restricta was identified most frequently in 221 cases (22.0%). M. globosa in 218 cases (21.7%), M. sympodialis in 118 cases (11.7%), M. furfur in 45 cases (4.5%), and M. dermatis in 19 cases (1.9%), respectively in the 26S rDNA PCR-RFLP pattern (p<0.001) (Table 4).
Among Malassezia yeasts cultured on the different body areas according to ages, M. globosa was identified as common species in the ages 1~10 (32.8%), 11~20 (28.9%), and 21~30 (32.3%) (p<0.0001) and M. restricta was identified as common species in the ages 41~50 (27.9%), 61~70 (31.5%), and 71~80 (24.0%) (p<0.0001). In the age 31~40, M. sympodialis was found as the most common species (24.6%) and M. restricta, 23.0% and M. globosa, 19.0% were observed. In the age 51~60 years, M. restricta and M. globosa were identified at the same rate of 24.2% (Table 4).
The results from each body site indicated that M. restricta was the most common species (56.8%) in the scalp. M. restricta, about 39.8% and M. globosa, 25.7% were found in the forehead, and M. restricta, 24.0% and M. globosa, 23.4% were identified in the cheek. On the other hand, M. globosa, 36.8% and M. sympodialis, 23.0% in the chest, M. sympodialis, 13.6% and M. globosa, 12.3% in the upper arm, and M. globosa, 7.7% in the thigh were isolated and identified (Table 5). Other species such as M. slooffiae, M. furfur, M. dermatis and M. obtusa were identified in a low frequency. Notably, M. dermatis, which was isolated from atopic dermatitis patients in Japan, was isolated and identified in 19 cases of the healthy skins. Interestingly, more than one Malassezia species was recovered from some plates. M. restricta+M. globosa were observed in 18 cases and M. restricta+M. sympodialis were in 11 cases (Fig. 3).
Malassezia yeasts are normal flora of the skin that are discovered in 75~80% of health subjects, but since its association with various skin disorders have been known, many studies have been conducted in the distribution of the yeasts. However, most of the studies have been focusing on morphological and biochemical analysis, which are time-consuming and subject to controversy, and the objective criteria are ambiguous.
Guého et al11 reclassified the Malassezia yeasts into 7 species (M. furfur, M. obtusa, M. globosa, M. slooffiae, M. sympodialis, M. pachydermatis, and M. restricta) based on morphological analysis and biochemical analysis, but recently 4 additional species (M. dermaits, M. japonica, M. nana, and M. yamatoensis) have been discovered on the basis of genetic analysis29-32, thereby classifying Malassezia yeasts into 11 species. However, because newly reported species with different genotypes are morphologically and biochemically identical or similar to the already known 7 species, the previous studies on the distribution of Malassezia yeasts cannot be pronounced to be accurate. Therefore, in recent studies many analytical equipments and software have been utilized for more accurate results18-32,36. RFLP method that was used in this study enables us to examine genetic variations through cleaving the amplified DNA with restriction enzymes and analyzing the patterns of the fragments. The method has many advantages in that it is less time-consuming, more accurate, and more cost-effective, and therefore can be utilized in rapid diagnosis and epidemiological studies24-28. The 26S rDNA, which was targeted in this study contains highly conserved base sequences and sufficient sequence variations for interspecies specific identification27,28. Also, it is quite compatible with morphological methods and appropriate for identification currently known Malassezia species, and requires only two restriction enzymes, Hha1, BstF51, and proven to be technically more simple than other molecular biology techniques. Therefore the authors opted for 26S rDNA PCR-RFLP method for accurate analysis in this study.
The frequency of Malassezia yeasts recovery depends on various factors such as age, sex, body sites, and other various internal and external factors and the result varies according to the difference in techniques of identification. Faergemann and Fredriksson37, in their study conducted on age group under 15, reported that the frequency of recovery increases with increase in age, and this phenomenon was attributed to the fact that sebaceous secretion peaks at adolescents. Bergbrant and Faergemann38 investigated the composition of skin lipid and the number of yeasts in the chest on 60 subjects that fall into the age group of 29~81, and proposed that increasing age is associated with decrease in the number of yeasts and the amount of lipid. Also, they reported that the culture rate is the highest in the age group of 20's and that the Malassezia yeasts are firmly established as the normal flora of the skin in the age group of over 20's. Lee et al12, in a study targeting age groups of 0~60 normal subjects, showed the highest culture rate in age group of 20's, and reported that age group of 20's have active sebaceous glands and Malassezia yeasts firmly exist as the normal flora, unlike children before adolescence. Accordingly, in our study, the culture rate was the highest in the ages 21~30 and 31~40 with 74.2%. This difference may be attributed to the fact that the secretion of androgen peaks during adolescence and therefore sebaceous secretion increases. However, the report by Kwon et al39 differed from other study results in that the recovery rate was the highest in older age groups. This may be attributable to the fact that other factors, such as hygiene, play a role in the growth of Malassezia yeasts, not only biological factors associated with sebum secretion.
It is well known that the yeasts are more widely distributed in the scalp, face and chest compared to areas such as the upper and lower limbs37. In our study, the culture rate was the highest in the scalp with 90%, followed by the forehead and chest with 81.3%, the cheek with 68.8%, the upper arm with 31.9%, and the thigh with 21.3%, which is accordance the site of occurrence of various Malassezia-related diseases such as pityriasis versicolor, seborrhaic dermatitis, and Malassezia folliculitis.
On 26S rDNA PCR-RFLP analysis, M. restricta and M. globosa were the most frequently discovered species with 22% and 21.7%, respectively. M. globosa showed high frequency in age 0~10, 11~20 and 21~30 compared to other age groups, and M. restricta and M. sympodialis in age 31~40, M. restricta in age group from 40 to 80, and an increase in M. restricta as age increases. Especially in age 0~10, M. globosa were the most predominant species whereas M. restricta were recovered in only 1 case. According to body site, M. restricta was more frequently recovered in the forehead and scalp while M. globosa and M. sympodialis was more frequently recovered in the chest. This observation was in accord with the fact that M. restricta and M. globosa are the normal flora of the forehead and anterior chest, respectively, in Koreans. However, in previous studies, it was reported that M. globosa was relatively more widely distributed than M. sympodialis on the chest of healthy Koreans12, while in this study M. globosa and M. sympodialis were the predominant species. Gupta and Kohli40 reported that in normal Japanese subjects M. globosa and M. sympodialis were the predominant species of the forehead and chest. Nakabayashi et al41 and Leeming et al42 reported that M. sympodialis were recovered in high rate, while M. globosa, M. sympodialis, and M. furfur were recovered in high frequency in the scalp and forehead. Also, Ashbee et al43 stated that colony A that corresponds to M. sympodialis were recovered predominantly in the chest and forehead. This disagreement may be attributable to such external factors as geographic distribution, and racial differences, different experimental techniques, the hygiene of the subjects, climate, and life styles. In this study, results may differ from previous studies12-15. It may be also attributable to the fact that molecular biological techniques were used. Especially in this study, M. dermatis, newly isolated Malassezia species from atopic dermatitis patient in Japan, was isolated and identified in 19 cases in healthy subjects. M. dermatis, first isolated by Sugita et al30, forms clusters with M. sympodialis on the phylogeny tree but shows dissimilarities between M. dermatis and M. sympodialis strain in their D1 and D2 regions of 26S, ITS1 at 1.2%, 10.0% and 10.3%, respectively, and thus is being recognized as a wholly new yeasts species, different from M. sympodialis. Also, because it shows identical characteristics with that of M. furfur on Tween utilization, catalase test and phenotypic analysis, they reported that M. dermatis cannot be distinguished from M. furfur on morphological and biochemical analysis. Because of these peculiarities, many researchers may have errored in classifying M. dermatis as M. sympodialis or M. furfur. Considering that the correlation between the degree of colonization of M. dermatis and the severity of atopic dermatitis is not yet known, and given the fact that it has been isolated in 19 cases of normal subjects in this study, further studies are probably necessary. Furthermore, 26S rDNA PCR-RFLP analysis which enables us to identify and differentiate formerly unidentified species are confirmed to identify these new species and may be useful in the diagnosis of Malassezia related diseases.
The common feature of many studies, including this study, is that Malassezia species is widely distributed the areas of high sebaceous secretion, and peaks during 20's, which is the period of highest sebum secretion. However, the distribution profile varies within the same body sites and same age groups depending on the researcher and the difference in the results of qualitative analysis may be attributable to the fact to various internal and external factors such as racial and geographic differences, difference in experimental techniques and inevitable errors in each research. Future studies may have to focus on comprehensive research that encompasses more diverse ethnic groups and wider age range. Efforts should also be made to develop more rapid and accurate diagnosis, and more sophisticated molecular techniques in order to accommodate more accurate identification of the yeasts, so that they may be implemented in the epidemiological studies of fungal diseases.
Figures and Tables
 | Fig. 126S rDNA PCR products of 11 Malassezia standard strains. In PCR to amplify the 26S rDNA, approximately 580 bp PCR band was identified in all of 11 standard colonies (Lane M: 100 bp DNA ladder, Lane 1: M. furfur, Lane 2: M. sympodialis, Lane 3: M. globosa, Lane 4: M. restricta, Lane 5: M. slooffiae, Lane 6: M. pachydermatis, Lane 7: M. japonica, Lane 8: M. nana, Lane 9: M. dermatis, Lane 10: M. obtusa, Lane 11: M. yamatoensis, Lane 12: negative control). |
 | Fig. 226S rDNA PCR-RFLP patterns that digested with Hha1 (A), BstF51 (B) of Malassezia standard strains. On analysis of PCR-RFLP of 26S rDNA using restriction enzymes Hha1, 9 different species, including M. furfur, M. globosa, M. restricta, M. slooffiae, M. pachydermatis, M. japonica, M. nana, M. obtusa, and M. yamatoensis showed interspecifically distinguishable restriction pattern but M. sympodialis and M. dermatis produced same pattern (A). In order to differentiate the two species, we used an additional restriction enzyme BstF51 to prove those two are indeed different species (B) (Lane M: 100 bp DNA ladder, Lane 1: M. furfur, Lane 2: M. sympodialis, Lane 3: M. globosa, Lane 4: M. restricta, Lane 5: M. slooffiae, Lane 6: M. pachydermatis, Lane 7: M. japonica, Lane 8: M. nana, Lane 9: M. dermatis, Lane 10: M. obtusa, Lane 11: M. yamatoensis). |
References
1. Janik MP, Heffernan MP. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Yeasts infection: Candidiasis, Pityriasis (Tinea) versicolor. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1822–1830.
2. Ahn KJ. Taxonomy of the genus Malassezia. Korean J Med Mycol. 1998. 3:81–88.
3. Ljubojević S, Skerlev M, Lipozencić J, Basta-Juzbasic A. The role of Malassezia furfur in dermatology. Clin Dermatol. 2002. 20:179–182.
4. Kanda N, Tani K, Enomoto U, Nakai K, Watanabe S. The skin fungus-induced Th1- and Th2-related cytokine, chemokine and prostaglandin E2 production in peripheral blood mononuclear cells from patients with atopic dermatitis and psoriasis vulgaris. Clin Exp Allergy. 2002. 32:1243–1250.

5. Ginarte M, Fabeiro JM, Toribio J. Confluent and reticulated papillomatosis (Gougerot-Carteaud) successfully treated with tacalcitol. J Dermatolog Treat. 2002. 13:27–30.

6. Yesudian P, Kamalam S, Razack A. Confluent and reticulated papillomatosis (Gougerot-Carteaud). An abnormal host reaction to Malassezia furfur. Acta Derm Venereol. 1973. 53:381–384.
7. Chowdhary A, Randhawa HS, Sharma S, Brandt ME, Kumar S. Malassezia furfur in a case of onychomycosis: colonizer or etiologic agent? Med Mycol. 2005. 43:87–90.

8. Gupta AK, Batra R, Bluhm R, Boekhout T, Dawson TL Jr. Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004. 51:785–798.
9. Devlin RK. Invasive fungal infections caused by Candida and Malassezia species in the neonatal intensive care unit. Adv Neonatal Care. 2006. 6:68–77.

10. Curvale-Fauchet N, Botterel F, Legrand P, Guillot J, Bretagne S. Frequency of intravascular catheter colonization by Malassezia spp. in adult patients. Mycoses. 2004. 47:491–494.

11. Guého E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie Van Leeuwenhoek. 1996. 69:337–355.

12. Lee YW, Yim SM, Lim SH, Choe YB, Ahn KJ. Quantitative investigation on the distribution of Malassezia species on healthy human skin in Korea. Mycoses. 2006. 49:405–410.

13. Guillot J, Gueho E, Lesourd M, Midgley G, Chevrier G, Dupont B. Identification of Malassezia species: a practical approach. J Mycol Med. 1996. 6:103–110.
14. Rodriguez-Valero S, Mesa LM, Gonzalez-Moran E, Delmonte ML, Robertiz S, Valero A. Phenotypic characterization of species of Malassezia in healthy skin of an university student population. Invest Clin. 2005. 46:329–335.
15. Bernier V, Weill FX, Hirigoyen V, Elleau C, Feyler A, Labrèze C, et al. Skin colonization by Malassezia species in neonates: a prospective study and relationship with neonatal cephalic pustulosis. Arch Dermatol. 2002. 138:215–218.
16. Morishita N, Sei Y, Sugita T. Molecular analysis of Malassezia microflora from patients with pityriasis versicolor. Mycopathologia. 2006. 161:61–65.

17. Sugita T, Tajima M, Tsubuku H, Tsuboi R, Nishikawa A. Quantitative analysis of cutaneous Malassezia in atopic dermatitis patients using real-time PCR. Microbiol Immunol. 2006. 50:549–552.

18. Senczek D, Siesenop U, Bohm KH. Characterization of Malassezia species by means of phenotypic characteristics and detection of electrophoretic karyotypes by pulsed-field gel electrophoresis (PFGE). Mycoses. 1999. 42:409–414.
19. Gupta AK, Boekhout T, Theelen B, Summerbell R, Batra R. Identification and typing of Malassezia species by amplified fragment length polymorphism and sequence analyses of the internal transcribed spacer and large-subunit regions of ribosomal DNA. J Clin Microbiol. 2004. 42:4253–4260.

20. Theelen B, Silvestri M, Guého E, van Belkum A, Boekhout T. Identification and typing of Malassezia yeasts using amplified fragment length polymorphism (AFLP), random amplified polymorphic DNA (RAPD) and denaturing gradient gel electrophoresis (DGGE). FEMS Yeast Res. 2001. 1:79–86.

21. Gandra RF, Simao RC, Matsumoto FE, da Silva BC, Ruiz LS, da Silva EG, et al. Genotyping by RAPD-PCR analyses of Malassezia furfur strains from pityriasis versicolor and seborrhoeic dermatitis patients. Mycopathologia. 2006. 162:273–280.

22. Gaitanis G, Velegraki A, Alexopoulos EC, Chasapi V, Tsigonia A, Katsambas A. Distribution of Malassezia species in pityriasis versicolor and seborrhoeic dermatitis in Greece. Typing of the major pityriasis versicolor isolate M. globosa. Br J Dermatol. 2006. 154:854–859.

23. Gemmer CM, DeAngelis YM, Theelen B, Boekhout T, Dawson TL Jr. Fast, noninvasive method for molecular detection and differentiation of Malassezia yeast species on human skin and application of the method to dandruff microbiology. J Clin Microbiol. 2002. 40:3350–3357.

24. Guillot J, Deville M, Berthelemy M, Provost F, Gueho E. A single PCR-restriction endonuclease analysis for rapid identification of Malassezia species. Lett Appl Microbiol. 2000. 31:400–403.

25. Makimura K, Tamura Y, Kudo M, Uchida K, Saito H, Yamaguchi H. Species identification and strain typing of Malassezia species stock strains and clinical isolates based on the DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Med Microbiol. 2000. 49:29–35.

26. Gaitanis G, Velegraki A, Frangoulis E, Mitroussia A, Tsigonia A, Tzimogianni A, et al. Identification of Malassezia species from patient skin scales by PCR-RFLP. Clin Microbiol Infect. 2002. 8:162–173.

27. Mirhendi H, Makimura K, Zomorodian K, Yamada T, Sugita T, Yamaguchi H. A simple PCR-RFLP method for identification and differentiation of 11 Malassezia species. J Microbiol Methods. 2005. 61:281–284.

28. Lee YW, Lim SH, Ahn KJ. The application of 26S rDNA PCR-RFLP in the identification and classification of Malassezia Yeast. Korean J Med Mycol. 2006. 11:141–153.
29. Sugita T, Kodama M, Saito M, Ito T, Kato Y, Tsuboi R, et al. Sequence diversity of the intergenic spacer region of the rRNA gene of Malassezia globosa colonizing the skin of patients with atopic dermatitis and healthy individuals. J Clin Microbiol. 2003. 41:3022–3027.

30. Sugita T, Takashima M, Shinoda T, Suto H, Unno T, Tsuboi R, et al. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J Clin Microbiol. 2002. 40:1363–1367.

31. Sugita T, Takashima M, Kodama M, Tsuboi R, Nishikawa A. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J Clin Microbiol. 2003. 41:4695–4699.

32. Hirai A, Kano R, Makimura K, Duarte ER, Hamdan JS, Lachance MA, et al. Malassezia nana sp. nov., a novel lipid-dependent yeast species isolated from animals. Int J Syst Evol Microbiol. 2004. 54:623–627.

33. Williamson P, Kligman AM. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol. 1965. 45:498–503.

34. Leeming JP, Notman FH, Holland KT. The distribution and ecology of Malassezia furfur and cutaneous bacteria on human skin. J Appl Bacteriol. 1989. 67:47–52.

35. Leeming JP, Notman FH. Improved methods for isolation and enumeration of Malassezia furfur from human skin. J Clin Microbiol. 1987. 25:2017–2019.

36. Sugita T, Tajima M, Takashima M, Amaya M, Saito M, Tsuboi R, et al. A new yeast, Malassezia yamatoensis, isolated from a patient with seborrheic dermatitis, and its distribution in patients and healthy subjects. Microbiol Immunol. 2004. 48:579–583.

37. Faergemann J, Fredriksson T. Age incidence of Pityrosporum orbiculare on human skin. Acta Derm Venereol. 1980. 60:531–533.
38. Bergbrant IM, Faergemann J. Variations of Pityrosporum orbiculare in middle-aged and elderly individuals. Acta Derm Venereol. 1988. 68:537–540.
39. Kwon HC, Kang SH, Kim HU. The distribution of Malassezia yeasts on normal human skin by culture study using the scrub-wash technique. Korean J Dermatol. 1999. 37:38–45.
40. Gupta AK, Kohli Y. Prevalence of Malassezia species on various body sites in clinically healthy subjects representing different age groups. Med Mycol. 2004. 42:35–42.

41. Nakabayashi A, Sei Y, Guillot J. Identification of Malassezia species isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med Mycol. 2000. 38:337–341.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download