Abstract
Background
Several workers have found that Malassezia are capable of suppressing cytokine release and downregulating the phagocytic function of monocytes. But lipid-depleted Malassezia furfur (M. furfur) extracts have also been shown to induce increased production of TNF-α, IL-6 and IL-1β in monocytes. We thought that the detergents in shampoos or soaps could change the composition of the lipid in the M. furfur cell wall.
Objective
We studied whether detergents affect the morphology of M. furfur and if the inflammatory cytokine profiles change in the monocytes treated with detergent-treated M. furfur.
Methods
Commonly used detergents such as sodium lauryl sulfate, ammonium lauryl sulfate and tween-80 were respectively added to the modified Leeming-Notman's media. M. furfur was cultivated in each media (detergent-added or untreated). Thereafter, the surface morphology of the yeast was evaluated by scanning and transmission electron microscopy. The cytokine profiles of monocytes, which were treated by M. furfur with or without detergents, were also evaluated.
Results
The detergent-treated M. furfur were similar to the lipid-extracted form of M. furfur on the electron microscopic study, with a recessed, withered surface and with thinner and rather electron transparent cell walls than the detergent-untreated M. furfur. The levels of TNF-α were higher in monocytes treated with detergent-treated Malassezia than that in the monocytes treated with the detergent-untreated Malassezia (p<0.05).
Conclusion
According to the findings in this study, it could be inferred that the detergents in shampoos or soaps affect the lipid layers of the Malassezia cell wall and these lipid-extracted Malassezia induce or aggravate some inflammatory conditions. But to correlate the relationship between detergents and Malassezia-associated diseases, in vivo experiments that will focus on short-term contact with detergents in real life conditions should be done.
The Malassezia species is one of the normal human cutaneous commensal flora, and it is related with various cutaneous diseases such as seborrheic dermatitis, tinea versicolor and Malassezia folliculitis1,2. It has been reported that the genus Malassezia has lipid-rich multiple layers on the cell wall3-5. It is thought that the lipid-rich layers play a critical role in physiologic and pathologic conditions. According to Kesavan et al6, Malassezia furfur (M. furfur) has the ability to modulate the proinflammatory and immunomodulatory response of human peripheral blood mononuclear cells and keratinocytes. It has been reported that Malassezia is capable of suppressing cytokine release and downregulating the phagocytic function of monocytes. Moreover, lipid-extracted M. furfur was shown to produce high levels of TNF-α, IL-6 and IL-1β in monocytes7,8, and it induced proinflammatory cytokine production via a TLR2-dependent mechanism9. It could be inferred that the detergents in shampoo and soap alter the composition of the lipid in the cell wall of M. furfur, and this may have some role in Malassezia-associated inflammatory dermatoses. In this study, we demonstrate that the detergents in shampoo and soap affect the morphology of M. furfur and the pro-inflammatory cytokine (i.e., TNF-α) profiles in monocytes that were treated with detergent-treated M. furfur.
M. furfur CBS 6001 (Centraalbureau voor Schimmelcultures, Utrecht, Netherlands) was used in these studies.
M. furfur was cultured for 3 days at 37℃ in modified Leeming-Notman medium (MLNM): 1% w/v bacteriological peptone (Difco™, BD, Sparks, MD, USA), 1% w/v glucose (Oxoid Limited, Hampshire, UK), 0.2% w/v yeast extract (Oxoid Limited), 0.8% w/v of desiccated ox bile (Oxoid Limited), 1% v/v of glycerol (Sigma-Aldrich, St. Louis, MO), 0.05% w/v of glycerol monostearate (VWR International Ltd., Leicestershire, UK), 2% v/v ml of olive oil (Sigma-Aldrich) and 1.5% w/v of agar No. 1 (Oxoid Limited). M. furfur was also grown in MLNM with detergent added [0.2% Tween 80, 0.1 or 0.2% sodium lauryl sulfate (SLS), and 0.1% or 0.2% ammonium lauryl sulfate (ALS)]. After cultivation, the yeast cells were suspended in PBS buffer (Medical & Biological Laboratories Co., Nagoya, Japan), and then they were washed three times. The cell suspensions were vortexed for 20 seconds to disperse the Malassezia clumps.
M. furfur was fixed for 30 min with 2% gluteraldehyde in 1×PBS, (pH=7.35 and at room temperature), and then the cells were washed three times and suspended in 1 mL 1×PBS. The scanning EM samples were filtered (or placed) onto a micropore filter. The filters with the sample on top were dehydrated in a graded series of ethanol solutions (50%, 75%, 95% and 100%) for 15 minutes in each solution, and this was followed by similar transfers into hexa-methyl-disilazane reagent solutions (50%, 75%, 95%) for 30 minutes for each solution, and this was followed by placing the filters in 100% hexa-methyl-disilazane reagent overnight. The scanning EM samples were gold coated and then viewed and photographed on a Cambridge Scanning electron microscope. The transmission EM samples were dehydrated in a series of graded ethanol solutions as described above, embedded in Epon (Hexion Specialty Chemicals, Columbus, OH, USA) and sectioned on a Sorvall MT6000 (RMC, Tucson, AZ, USA). Thin sections (75 µm) were stained with uranyl acetate, and then they were viewed and photographed on a Jeol XC100 at 80-kV.
The human monocytic cell line U-937 (American Type Culture Collection, Rockville, MD, USA) was cultured in RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (JRC Biosciences, Lenexa, KS, USA), 0.6 mg/ml L-glutamate (Wako Pure Chemical Co., Osaka, Japan), 100 U/ml penicillin (Banyu Pharmaceutical Co., Tokyo, Japan) and 100 mg/ml streptomycin (Meiji Seika Co., Tokyo, Japan) under a humidified atmosphere containing 5% CO2 at 37℃ for 7 days. After 7 days in culture, the adherent cells were washed three times with pre-warmed RPMI 1640, and then they were supplemented with 60 mg/ml of penicillin, 100 mg/ml of streptomycin, 20 mM HEPES and 5% (v:v) heat-inactivated fetal calf serum. The monocytes were resuspended at 1×106 cells/ml in culture medium.
The monocytes were cultured with 10 or 30 µg/ml of detergent-untreated or detergent-treated M. furfur for 24 h for performing ELISA. As a positive control, 10 µg/ml of lipopolysaccharide (LPS; from Escherichia coli [Sigma, Poole, UK]) was also cultured with the monocytes. After the overnight incubation, the cell-free supernates were used for ELISA assay.
A 96-well plate (Becton, Dickinson and Company, NJ, USA) was coated with anti-human TNF-α monoclonal antibody (Pharmingen, CA, USA). One hundred microliter aliquots of the mononuclear cell supernantants were added to the respective wells and a secondary mouse anti-human TNF-α monoclonal antibody was then added. The antibody-cytokine-antibody complex was detected by a reaction with biotinylated anti-mouse antibodies and horseradish-peroxidase-avidin conjugate (Vector Laboratories, Peterborough, UK). The color reaction was detected by adding orthophenylenediamine (OPD; Sigma, Dorset, UK) and the reaction stopped with 2·5 M of H2SO4. The absorbances at 450 nm were determined by using an ELISA plate reader (Molecular Devices Co. Ltd., USA).
The detergent-untreated M. furfur showed the normal pleomorphic structure on both the scanning and transmission EM micrographs. On the scanning EM, the surface of the untreated M. furfur appeared smooth and rounded with fimbriae present, whereas the Tween 80-treated M. furfur demonstrated a recessed, withered surface (Fig. 2). On the transmission EM, the untreated M. furfur had a cell wall that consisted of an outer lamellar layer, an inner indentation layer and a "membrane-like" electron-transparent middle layer between two electron-dense layers. However, the cell wall of the detergent-treated M. furfur was thinner and more electron-transparent than that of the untreated M. furfur (Figs. 2, 3).
For the detergent-untreated M. furfur, the mean specific activity of the immunochemical TNF-α was 241 pg/ml and 268 pg/ml in the monocytes cultured with 10 µg/ml and 30 µg/ml of the detergent-untreated M. furfur, respectively For the monocytes cultured with the detergent-treated M. furfur, the levels of TNF-α ranged from 157 to 4,062.5 pg/ml. The LPS-treated monocytes as a positive control showed a mean specific TNF-α activity of 2,347.5 in the monocytes treated with 10 µg/ml of M. furfur and 2,628.5 pg/ml in those with 30 µg/ml. The levels of TNF-α in the moncytes cultured with 10 µg/ml and 30 µg/ml of Malassezia were 604.5 and 1,018 in tween 80, 1,367 and 1,578.5 in 0.1% SLS, 1,941.5 and 1,773.5 in 0.2% SLS, 2,038.5 and 2,606.5 in 0.1% ALS, and 3,089 and 4,062.5 in 0.2% ALS. The maximum cytokine production was detected in the 0.2% ALS treated monocyte group, and this was significantly greater than the positive control (Fig. 4).
The levels of TNF-α by the monocytes in response to the detergent-treated and untreated M. furfur were analyzed. The detergent-treated M. furfur induced a higher level of TNF-α than did the untreated M. furfur, with statistical significance (p<005). There was also a positive correlation between the induction of TNF-α and the concentrations of SLS and ALS (p<0.05).
In humans, M. furfur usually reside in the sebum-rich areas of the body1. To survive in a normal environment, M. furfur has its own protective mechanism against host defenses10. The melanin-like pigment allows the yeast to be less susceptible to the reactive oxygen species generated during the immunological response, and the lipases, phospholipases and hydrolases of yeasts are essential for providing the lipids that are required for growth11-14.
The cell wall of Malassezia is very thick in comparison with other yeasts and it constitutes 26% to 37% of the cell volume15. The cell envelop of M. furfur is composed of 3 layers: the outer lamellar layer on the surface, the inner indentation layer closely attached to the plasma membrane and the "membrane-like" electron-transparent middle layer, which is enclosed by two electron-dense layers3,4,16. The outer layer, which seems to be equivalent to the capsule, is a unique structure of Malassezia and it is thought to contain lipid components. There is an inconsistently visible intermediate zone that separates the main layers. The main layers may be further lamellated, thus revealing a multi-layered substructure3. The Malassezia yeast cell wall is composed of an unusually high lipid content (15~20%). This is a feature that is very distinctive from other yeasts such Candida albicans (1~2%) and Saccharomyces cerevisiae (1~2%)1.
The ways in which Malassezia interacts with the host immune system is, in many ways, paradoxical17,18. Several researchers have insisted that Malassezia is able to upregulate phagocytic cells and thus this provides enhanced protection against bacterial and tumor cell challenges in animals1,19,20. Yet other studies have demonstrated that Malassezia is also capable of downregulating the immune system7,19. When various preparations of Malassezia were co-incubated with monocytes, the levels of IL-1β released by the monocytes were significantly lower than those of the negative control17. This apparent contradictory behavior may be related to the lipid-rich capsule-like layer that surrounds the yeast cells. Removal of the capsule-like layer reverses the suppression of cytokine production by monocytes7. Kesavan et al6 have examined the effect of the Malassezia species with and without the capsular layer on the cytokine production by monocytes. As a result, the levels of IL-1α, IL-6 and TNF-α production were lower in the Malassezia with the capsular layer, suggesting that the capsule suppresses the release of proinflammatory cytokine and it prevents an inflammatory response7. Moreover, Oh et al9 revealed that when M. furfur lipids were removed from the cell wall, the capacity to activate primary human monocytes was restored, and this was correlated with the induction of proinflammatory cytokine production via a TLR2-dependent mechanism. In addition, the mechanism by which M. furfur lipids inhibited cytokine production in monocytes was found to be via down-regulation of the TLR2 expression9. Therefore, the ability of M. furfur to exist in a homeostatic environment may be partly due to the expression of immune inhibitory lipids on its cell wall20. However, the exact lipid that is responsible for the immune inhibition has not yet been established.
In shampoo and soap, there are various ingredients such as detergent, viscosity control agents, preservatives, fragrances, dye etc21,22. Detergents are used for skin cleansing and as wetting and emulsifying agents. According to the hydrophilic polar group, detergents are classified as anionic, cationic, amphoteric and nonionic. The anionic surfactant in most products has outstanding cleansing properties. These days, various anionic surfactants are being used together with other ingredients to optimize the cleansing properties22,23. We hypothesized that the detergents in shampoo or soap can alter the composition of the lipid in the cell wall of M. furfur, as in lipid extraction, and this could cause Malassezia-associated inflammatory dermatoses. In this study, we used the commonly used anionic detergents such as, SLS, ALS and Tween 80, and these detergents changed the morphology and structure of M. furfur by altering the cell wall lipid layer, as was seen on the EM study. M. furfur cultured in detergent-treated media showed a more undulating and transparent cell wall than did the M. furfur cultured in regular media. M. furfur grown in media with detergents had a resemblance to the lipid-extracted form, and it also induced greater TNF-α in the monocytes than did those M. furfur grown in regular media. The level of TNF-α varied according to the different concentrations and types of detergents. The M. furfur grown in 0.2% ALS, induced more TNF-α than was induced by the positive control with LPS (p<0.05). The production of TNF-α was greater for the 0.2% SLS than for the 0.1% SLS, and this was greater for the 0.2% ALS than for the 0.1% ALS, all with statistical significance (p<0.05). Yet the concentration of Malassezia seemed to be statistically irrelevant.
It is possible that for the individuals with Malassezia-associated inflammatory dermatoses such as seborrheic dermatitis, the physiology of the normal commensal yeast is altered. Thus, the presence of abnormal rendering yeast can induce inflammatory responses9,18. M. furfur grown in media with detergents induced greater proinflammatory cytokine in the monocytes than did those M. furfur grown in regular media, and detergents influenced the morphology of M. furfur by alternating the cell wall lipid layer, as was seen on the EM. The detergents in shampoo and soap modify the lipid component of the cell wall and they transform the morphologic features, along with inducing an inflammatory effect. Thus, it could be inferred that the detergents in shampoo and soap have some role in Malassezia-associated inflammatory dermatoses.
In our experiments, Malassezia grew in detergent-added media for 3 days. However, the detergents used for daily skin cleansing are in contact with the skin for only short periods of time, and it is not reasonable to soak human skin in detergents for a long period. Besides, the shampoo and soap used nowadays contain various detergents that have less toxic effect, and so further research is needed on other detergents that may affect Malassezia furfur. Accordingly, it is uncertain that detergents can not only cause, but also aggravate Malassezia-associated inflammatory dermatosis by making changes in the morphology and immunomodulatory function of M. furfur. However, we think that in vivo experimentation and also assessing the molecular changes in the lipid component of the cell wall after short-term contact with detergents are required to establish the precise relationship between the effect of detergent and the Malassezia-associated inflammatory dermatoses.
Figures and Tables
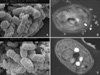 | Fig. 2Scanning and transmission electron microscopic findings of the detergent-untreated and tween-80 treated M. furfur. (A) Scanning EM of the detergent-untreated M. furfur shows a smooth, round surface with fimbriae. (B) The transmission EM shows outer lamellar layers (arrowhead), an inner plasma membrane (arrow) and intermediate multiple layers. (C) M. furfur cultured in the tween-80 added media shows recessed and withered irregular surfaces on the scanning EM. (D) As compared with the untreated M. furfur, the cell wall of the M. furfur cultured in the tween-80 added media is much thinner and more transparent on the transmission EM. |
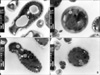 | Fig. 3Morphological alteration of the ALS-treated M. furfur. (A) Transmission electron microscopic findings of the M. furfur cultured in regular media. (B) The transmission electron microscopic findings of the M. furfur cultured in 0.2% ALS added media. As compared with the untreated M. furfur, the cell wall of the M. furfur cultured in 0.2% ALS added media is much thinner and more transparent. |
 | Fig. 4The effect of detergent on the M. furfur-induced TNF-α production of monocytes (as detemined by ELISA). As compared with the M. furfur from the non-treated media, the detergent-treated M. furfur induced greater TNF-α in the monocytes. MLNM: modified Leeming-Notman media, SLS: sodium lauryl sulfate, ALS: ammonium lauryl sulfate, LPS: lipopolysaccharide. |
References
1. Ashbee HR, Evans EG. Immunology of diseases associated with Malassezia species. Clin Microbiol Rev. 2002. 15:21–57.

2. Gupta AK, Batra R, Bluhm R, Boekhout T, Dawson TL Jr. Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004. 51:785–798.

3. Mittag H. Fine structural investigation of Malassezia furfur. II. The envelope of the yeast cells. Mycoses. 1995. 38:13–21.

4. McDaniel DH, Welton WA. Scanning electron microscopic evaluation of tinea versicolor. Effects of treatment with miconazole nitrate and clotrimazole. Arch Dermatol. 1984. 120:1057–1058.

5. Borgers M, Cauwenbergh G, Van de Ven MA, del Palacio Hernanz A, Degreef H. Pityriasis versicolor and Pityrosporum ovale. Morphogenetic and ultrastructural considerations. Int J Dermatol. 1987. 26:586–589.
6. Kesavan S, Walters CE, Holland KT, Ingham E. The effects of Malassezia on pro-inflammatory cytokine production by human peripheral blood mononuclear cells in vitro. Med Mycol. 1998. 36:97–106.

7. Kesavan S, Holland KT, Ingham E. The effects of lipid extraction on the immunomodulatory activity of Malassezia species in vitro. Med Mycol. 2000. 38:239–247.

8. Ishibashi Y, Sugita T, Nishikawa A. Cytokine secretion profile of human keratinocytes exposed to Malassezia yeasts. FEMS Immunol Med Microbiol. 2006. 48:400–409.

9. Oh C, Kim A, Kuo I, Krutzik SR, Liu PT, Sieling PA, et al. Lipids on the Malassezia furfur cell wall inhibit proinflammatory cytokine production in human monocytes by downregulating the toll-like receptor 2: immunomodulatory role of Malassezia furfur. J Invest Dermatol. 2005. 124:A127.
10. Ashbee HR. Update on the genus Malassezia. Med Mycol. 2007. 45:287–303.
11. Plotkin LI, Squiquera L, Mathov I, Galimberti R, Leoni J. Characterization of the lipase activity of Malassezia furfur. J Med Vet Mycol. 1996. 34:43–48.
12. Brunke S, Hube B. MfLIP1, a gene encoding an extracellular lipase of the lipid-dependent fungus Malassezia furfur. Microbiology. 2006. 152:547–554.

13. Mayser P, Scheurer C, Papavassilis C, Grunder K. Hydrolase activity of 150 Malassezia furfur isolates of different clinical origin. Mycoses. 1996. 39:225–231.

14. Riciputo RM, Oliveri S, Micali G, Sapuppo A. Phospholipase activity in Malassezia furfur pathogenic strains. Mycoses. 1996. 39:233–235.
15. Keddie FM, Barajas L. Quantitative ultrastructural variations between Pityrosporum ovale and P. orbiculare based on serial section electron microscopy. Int J Dermatol. 1972. 11:40–48.

16. Faergemann J, Borgers M. The effect of ketoconazole and itraconazole on the filamentous form of Pityrosporum ovale. Acta Derm Venereol. 1990. 70:172–176.
17. Suzuki T, Tsuzuki A, Ohno N, Ohshima Y, Yadomae T. Enhancement of IL-8 production from human monocytic and granulocytic cell lines, THP-1 and HL-60, stimulated with Malassezia furfur. FEMS Immunol Med Microbiol. 2000. 28:157–162.

18. Donnarumma G, Buommino E, Baroni A, Auricchio L, De Filippis A, Cozza V, et al. Effects of AV119, a natural sugar from avocado, on Malassezia furfur invasiveness and on the expression of HBD-2 and cytokines in human keratinocytes. Exp Dermatol. 2007. 16:912–919.

19. Watanabe S, Kano R, Sato H, Nakamura Y, Hasegawa A. The effects of Malassezia yeasts on cytokine production by human keratinocytes. J Invest Dermatol. 2001. 116:769–773.

20. Saadatzadeh MR, Ashbee HR, Cunliffe WJ, Ingham E. Cell-mediated immunity to the mycelial phase of Malassezia spp. in patients with pityriasis versicolor and controls. Br J Dermatol. 2001. 144:77–84.

21. Lochhead RY. Formulating conditioning shampoos. Cosmetics and Toiletries. 2001. 116:55–66.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download