Abstract
Background
Angioimmunoblastic T-cell lymphoma (AITL) is a complex lymphoproliferative disorder and often mimics a viral infection with frequent skin involvement. Epstein-Barr virus (EBV) and human herpes virus (HHV)-6 are reported to be associated with AITL, but there are conflicting results.
Methods
We reviewed the clinical, histological and immunophenotypical features of 19 cases of AITL. Among them, 11 lymph node biopsies of AITL were examined for HHV-6, -7, and -8 by polymerase chain reaction (PCR) using virus-specific primers. In situ hybridization of EBV early region RNA (EBER) was performed and T cell receptor (TCR) gene rearrangement was also investigated in some cases.
Results
Among these 19 cases, maculopapular, plaque or nodular skin lesions accompanied AITL in 12 cases. Clonal TCR gene rearrangement was seen in 8/9 cases tested. EBER in situ hybridization was positive in 8 cases (57.1%). Among 7 cases with skin biopsies, five cases were consistent with cutaneous involvement of AITL, 1 case was a drug eruption, and the other case was Kaposi's sarcoma. Except a HHV-8 (+) case who also had Kaposi's sarcoma, all of these cases were negative for HHV-6, -7 and -8.
Conclusion
Skin manifestation seems to be a cardinal component of AITL, be it in the context of presentation, progression or recurrent disease. Recognition of clinicopathological features of skin lesions in AITL as diagnostic clues should be stressed among dermatologists. The lack of HHV-6, -7 and -8 in lymph node biopsy of AITL argues against a pathogenic role for HHVs in AITL.
Angioimmunoblastic T cell lymphoma (AITL) is a complex lymphoproliferative disorder associated with disordered immunoregulation with both hyperactivity of the immune system (hypergammaglobulinemia, autoimmune phenomena) and immunodeficiency, such as association with EBV or HHV infection1.
As suggested by its former name, angioimmunoblastic lymphadenopathy with dysproteinemia (AILD)2, AITL is characterized by a polymorphic nature including fever, skin eruptions, polyclonal hypergammaglobulinemia, generalized lymphadenopathy and hepatosplenomegaly3. AILD was formerly considered an exaggerated immune response such as pseudolymphoma2. However, AILD has been recognized as a distinct entity and AITL is a specific form among mature peripheral T cell lymphoma (PTCL) in REAL/WHO classification4.
In previous reports, skin lesions occurred in 40% to 50% of AITL cases, but the skin pathology was reported to be merely nonspecific5-8. Because of the rarity of AITL and the near absence of skin biopsies, the skin lesions have not been clarified in the dermatologic literature7,8.
As to viral pathogenesis of AITL, in addition to Epstein-Barr virus (EBV)9, several studies investigated the association of HHV-6, HHV-7 and HHV-8 without convincing data10,11. A recent study insisted that HHV-6 and EBV have a pathogenic role in AITL12. We speculate that viral pathogenesis of AITL may be related to common skin involvement or other systemic symptoms of AITL because the clinical presentation of AITL often mimics viral exanthem. In addition, pityriasis lichenoides (PL) can overlap with mycosis fungoides (MF), and PL is reportedly related to HHV-6 or -7. The early stage of MF and other lymphoproliferative disorders is also reported to be related to HHV-611. Therefore, we investigated the presence of HHV-6, -7, and -8 in the 11 lymph node biopsies of AITL compared to other cutaneous lymphomas and peripheral T cell lymphomas, unspecified (PTCL) of lymph nodes.
Lymph-node biopsies diagnostic of AITL and dating from 1998~2004 were found. The clinical, histopathologic and immunophenotypical features of the cases were analyzed. A total of 19 cases of AITL were collected and lymph node AITL cases were reviewed using histology and immunohistochemistry. We investigated these 11 lymph node tissues by hetero-duplex polymerase chain reaction (PCR) for HHV-6, -7, and -8 DNA and by in situ hybridization of EBV early region RNA (EBER). T cell receptor (TCR) gene rearrangement was also investigated in the remaining 6 lymph node biopsy samples and 3 skin biopsy samples. For comparison, we also investigated the presence of HHV-6, -7, and -8 in 2 cases of cutaneous anaplastic large cell lymphomas (ALCL), 20 cases of MF, 9 cases of pityriasis lichenoides (PL) chronica, 8 cases of acute PL, 4 cases of unclassified PL, 2 cases of cutaneous NK T cell lymphomas (NKTL), 16 cases of lymph node PTCL, 3 normal skin controls and 2 normal lymph nodes.
The patient population comprised 12 men and 7 women with a male to female ratio of 1.7:1. Mean onset age was 55.3 years in men and 60.3 years in women with a range of 23 to 73 years. Although the most common first presentation of the disease was generalized lymphadenopathy (LAP), maculopapular skin eruption with fever was the first manifestation in 4 cases (Table 1).
Excisional biopsy samples of neck lymph nodes typically showed a tumor composed of lymphoid cells with proliferation of arborizing vessels and total effacement of nodal architecture. Plasma cells (12 cases) and some eosinophils (1 case) were infiltrating. The cells were usually small to medium in size (15 cases), but 4 cases showed dense infiltration of medium to large pleomorphic cells. Most of the lymphoid cells were positive for CD3, CD4 and CD21, with partial positivity for CD8 and CD30. CD20+ B cells were mixed. Proliferation of arborizing vessels and red blood cell extravasation were observed. Clonal TCR gene rearrangement was seen in 8/9 cases tested. Eight cases (57.1%) among the biopsy samples of involved lymph nodes were positive for EBER.
Overall, skin lesions accompanied 12 cases (63.2%) of AITL (Table 2). The nature of the skin lesions and the timing of skin lesion appearance were as follows: maculopapular eruptions during the course of disease (Case 3, Case 9, Case 10), multiple patches on the back and abdomen during the course of disease in 61-year-old man (Case 7). Case 2 was a 71-year-old male who had no skin lesions initially but had maculopapular eruption heralding the recurrence of AITL, four years after complete remission by chemotherapy. Lymph node biopsy showed clonality of both TCR and B cell receptor (BCR) and the cells were pleomorphic, medium and large cells. Skin biopsies were not obtained from these five cases, but skin involvement of AITL was clinically diagnosed. After chemotherapy, the skin lesions improved in all five cases.
The other 7 cases with skin lesions underwent skin biopsies. One case was patch stage Kaposi's sarcoma (Case 13) in a 60-year-old woman. She was HHV-8 (+) in AITL-lymph nodes, blood and several red plaques on her hands, which were diagnosed as Kaposi's sarcoma. Lymph node biopsies were negative for HHV-6, HHV-7 and EBER. Other skin manifestations in case 13 were facial edema, livedo reticularis and drug eruption-like maculopapular eruption after 6 cycles of combination chemotherapy with etoposide, methylprednisolone, and cisplatin. After that, complete remission (CR) was maintained for 17 months but no further follow-up was possible. In Case 11, a 42-year-old male presented with fever, lymphadenopathy, and anemia. During the diagnosing process, he developed red papules and patches with a few infiltrated plaques. Under the clinical impression of viral exanthem, a skin biopsy showed superficial and deep small- to medium-sized lymphocytic infiltrates. Some large atypical cells were seen. These skin lesions were suggestive of the diagnosis of AITL. Lymph node histology was similar and EBER was positive in the lymph node biopsy. The skin lesions of the other 5 cases were maculopapular to nodular eruption. Clinical impression of the 7 cases with available skin biopsies were as follows: viral exanthem (5 cases), secondary lymphoma (1 case), maculopapular eruption (6 cases), plaque to nodular eruption (1 case), symmetricity (6 cases), and itching (4 cases). Histologically, skin biopsy samples showed superficial and deep lymphocytic infiltration to atypical mononuclear cell infiltration. The diverse histopathologic findings are shown in Table 3. Two cases (Case 5 and Case 6) revealed monoclonal TCR gene rearrangement. Although the perivascular lymphoid infiltrates were relatively scarce and appeared blended on first glance, multiple sections and clonal TCR gene rearrangement helped to diagnose specific lesions composed of lymphomatous infiltration. Clinicopathological correlation confirmed 'skin involvement of AITL' in 5 cases and maculopapular drug eruption in 1 case (Case 15). Three cases (3/5) of 'skin involvement of AITL' died of disease within 3 years of diagnosis. In the remaining 2 cases, further follow-up was not possible.
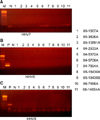
Normal skin controls and normal lymph nodes were all negative for HHV-6, -7 and -8. Paraffin blocks of 11 cases of AITL were available for virus studies. All cases were negative for HHV -6, -7, and -8 (Fig. 1). In comparison, HHV-6 DNA was found in 2 cases (25%) of acute PL (male to female 7:1, mean age 17.6 years) and in 2 cases (10%) of MF. Cutaneous ALCL, PL chronica, cutaneous NKTL, and lymph node PTCL were negative for HHV-6. As for HHV-7 and HHV-8, all samples were negative except one AITL case who presented with cutaneous Kaposi's sarcoma.
AITL is a biologically and clinically heterogeneous entity whose true nature remains to be clarified1. Histology of lymph node is often diagnostic, showing partial effacement of normal architecture by polymorphic inflammatory infiltrates of large immunoblasts, small lymphocytes, plasma cells, eosinophils, histiocytes and marked vascular proliferation2-4.
Skin involvement of AITL is frequent, as seen in our series, but underestimated due to lack of suspicion. Jaffe et al4 included AITL in T-cell and NK-cell lymphomas with frequent or constant cutaneous involvement. Although the diagnosis in LN is characteristic and the skin is frequently involved, the skin lesions are not well characterized in the dermatologic literature. In addition, the skin lesions in AITL have been regarded as nonspecific in most cases. A cutaneous eruption was observed in up to 50% of patients with AITL7. Skin lesions can precede, occur simultaneously, or develop after the diagnosis of the disease7,8. In our study, skin lesions were nonspecific, generalized, maculopapular eruptions mimicking a viral exanthem or a drug eruption. However, a wide range of skin lesions has been described including papules, plaques, nodules, erosions, urticarial or purpuric eruptions or diffuse erythema5-8.
Our study showed that the cells in the skin biopsy were not atypical. In comparison to superficial perivascular lymphocytic infiltration in drug or viral exanthem, cutaneous AITL showed superficial and deep lymphohistiocytic infiltrate with proliferation of vessels. In cases of non-diagnostic skin pathology, clonal TCR gene rearrangement was helpful for diagnosis. Since skin involvement of lymphomas is a poorer sign, accurate and prompt diagnosis of cutaneous involvement of AITL showing equivocal clinicopathologic findings should be made by clinical, immunohistochemical and molecular analysis.
Infectious diseases and agents have been reported to be associated with AITL, especially lymphotrophic viruses such as EBV. However, Brown et al7 reported remarkably heterogeneous EBV expression in cutaneous lesions of AITL. HHV-6 and -8 were isolated from the peripheral blood of AITL patients, and a possible role for these viruses in AITL has emerged from serologic and molecular studies. In a case report of AITL with disseminated HHV-6 infection in a patient with acute myeloblastic leukemia, the authors insisted that immunosuppression associated with AML and the additional iatrogenic immunosuppression predisposed the patient to reactivated HHV-6 infection10. However, Luppi et al9,11 revealed an association with HHV-6 and HHV-8 in only some cases. Another study also concluded that there was no significant association between T-cell clonality and HHV-6 or EBV infection, although lymph node biopsies revealed 22% of lymphotropic viral genome of HHV-6, 40% of EBV, but none of HHV-8. In this background of uncertain association with HHVs and because skin lesions in AITL cases can mimic viral exanthem and HHVs are known to be associated with viral exanthem, pityriasis or skin lymphomas, the association of HHV-6, -7, and -8 with AITL should be investigated by the more accurate method of hetero-duplex PCR. HHV-7, a beta-herpesvirus genetically close to HHV-6, utilizes CD4 as a receptor on the lymphocyte surface11. In our study, no association of HHVs with AITL was seen compared to other lymphomas. One case of HHV-8 (+) may be coincidental or related to the coexistence of Kaposi's sarcoma. The heterogeneity and variability of these viruses in previous studies and in our study suggest that these viral infections are not primary events in the pathogenesis of AITL.
Dermatologists should be aware of AITL and include skin lesions of AITL among the differential diagnoses of fever of unknown origin with generalized skin rash13-15. As the skin lesions closely mimic a benign condition and the skin biopsies of skin lesions of AITL are not malignant, clonality testing with clinical suspicion is crucial for early and accurate diagnosis and can prevent mistreatment or confusion of treatment policy.
In conclusion, AITL is not associated with HHVs. Skin eruptions accompanied by AITL are frequent and are not nonspecific, but rather are a cardinal component of AITL. Symmetric maculopapular, nonspecific-looking skin manifestation may indicate diagnosis of this unique T cell lymphoma.
Figures and Tables
References
1. Vrsalovic MM, Korac P, Dominis M, Ostojic S, Mannhalter C, Kusec R. T- and B-cell clonality and frequency of human herpes viruses-6, -8 and Epstein Barr virus in angioimmunoblastic T-cell lymphoma. Hematol Oncol. 2004. 22:169–177.

2. Weiss LM, Jaffe ES, Liu XF, Chen YY, Shibata D, Medeiros LJ. Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Blood. 1992. 79:1789–1795.

3. Dogan A, Attygalle AD, Kyriakou C. Angioimmunoblastic T-cell lymphoma. Br J Haematol. 2003. 121:681–691.

4. Jaffe ES, Harris NL, Stein H, Vardiman JS, editors. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. 2001. Lyon, France: IARC Press.
5. Schmuth M, Ramaker J, Trautmann C, Hummel M, Schmitt-Graff A, Stein H, et al. Cutaneous involvement in prelymphomatous angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1997. 36:290–295.

6. Martel P, Laroche L, Courville P, Larroche C, Wechsler J, Lenormand B, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000. 136:881–886.

7. Brown HA, Macon WR, Kurtin PJ, Gibson LE. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. J Cutan Pathol. 2001. 28:432–438.

8. Bernstein JE, Soltani K, Lorincz AL. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1979. 1:227–232.

9. Luppi M, Marasca R, Barozzi P, Artusi T, Torelli G. Frequent detection of human herpesvirus-6 sequences by polymerase chain reaction in paraffin-embedded lymph nodes from patients with angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Leuk Res. 1993. 17:1003–1011.

10. Daibata M, Ido E, Murakami K, Kuzume T, Kubonishi I, Taguchi H, et al. Angioimmunoblastic lymphadenopathy with disseminated human herpesvirus 6 infection in a patient with acute myeloblastic leukemia. Leukemia. 1997. 11:882–885.

11. Luppi M, Torelli G. The new lymphotropic herpesviruses (HHV-6, HHV-7, HHV-8) and hepatitis C virus (HCV) in human lymphoproliferative diseases: an overview. Haematologica. 1996. 81:265–281.
12. Zhou Y, Attygalle AD, Chuang SS, Diss T, Ye H, Liu H, et al. Angioimmunoblastic T-cell lymphoma: histological progression associates with EBV and HHV6B viral load. Br J Haematol. 2007. 138:44–53.

13. Jayaraman AG, Cassarino D, Advani R, Kim YH, Tsai E, Kohler S. Cutaneous involvement by angioimmunoblastic T-cell lymphoma: a unique histologic presentation, mimicking an infectious etiology. J Cutan Pathol. 2006. 33:6–11.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download