Abstract
Merkel cell carcinoma is a rare aggressive primary cutaneous neuroendocrine skin cancer arising from the basal epidermis. Although the majority of patients with non-melanoma skin cancer are cured by local treatment, patients with Merkel cell carcinoma have a poor outcome characterized by locoregional and distant relapse. No standard treatment protocol for Merkel cell carcinoma exists. But, there is mounting evidence that combined treatment, incorporating adjuvant radiotherapy, improves the outcome (locoregional control and disease-free survival) compared with surgery alone in most patients. Herein we report a case of Merkel cell carcinoma treated with wide local excision and post-operative radiotherapy.
Merkel cell carcinoma (MCC), also referred as neuroendocrine or trabecular carcinoma, is an uncommon malignancy originally described by Toker in 1972. Although the exact origin of the Merkel cell is unknown, it probably arises from neuroendocrine cells between the basal epidermis and then grows vertically into the dermis and subcutaneous tissue1. It usually arises in the head and neck area and extremities of elderly people as a nodule or plaque lesion. Cases of MCC have a poor outcome characterized by locoregional and distant relapse. It has been reported that 34% of affected patients die because of the tumor. Recent evidences suggest that surgery and adjuvant locoregional radiotherapy may produce a better disease-free survival compared with surgery alone2. Herein we present a case of Merkel cell carcinoma treated with wide excision and adjuvant local radiotherapy.
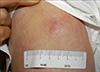
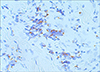
A 73-year-old man had a 2-month history of a rapidly growing painless mass on the left upper arm. Skin examination revealed an erythematous mass measuring 2.5×2.5 cm on the left upper arm (Fig. 1). There was no regional lymphadenopathy. General physical examination and laboratory tests revealed normal findings. Punch biopsy was taken and histopathological examination revealed the tumor was located in the deep dermis, partly extending into subcutaneous tissue. The tumor cells were arranged in dense cohesive sheets with a variable trabecular pattern. The individual cells had a monomorphous nature with round to oval, vesicular nuclei and scanty cytoplasm (Fig. 2). Mitotic figures were not seen. Immunohistochemically, positive results for neuron-specific enolase (NSE), chromogranin,synaptophisin and cytokeratin 20 were obtained. Leucocyte common antigen and S-100 were negative. Especially, staining for cytokeratin 20 showed perinuclear dot-like patterns (Fig. 3). Chest and abdomino-pelvic CT scan did not show abnormal findings. Whole body PET also showed normal findings. According to these findings, the diagnosis of Merkel cell carcinoma was made. The tumor was excised up to the muscle fascia with a 3 cm margin, and repair with full thickness skin graft was performed. And axillary lymph node dissection (7 lymph nodes) based on the intraoperative lymphangiographic finding was performed. But tumor cells were not found in axillary lymph node sections.
The patient underwent post-operative radiotherapy on the lesion and left axillary node area for 4 weeks (25 times, total 5,000 cGy). After 2.5 years, the patient is still in state of complete remission with no sign of disease recurrence in both the whole body PET and CT scan.
Merkel cell carcinoma (MCC) typically presents as a solitary dome-shaped, deep red to violaceous nodule or indurated plaque and tends to have a shiny surface, often with overlying telangiectasia3. The overlying skin is usually intact. Histologically, anastomosing cords and strands of neoplastic cells are seen in the dermis and subcutis, but not in the epidermis4. The individual cells are monomorphous with round vesicular nuclei and scanty, ill-defined cytoplasm. Ultrastructurally the tumor cells are characterized by the presence of paranuclear filament whorls and membrane-bound neurosecretory granules approximately 100~150 nm across4. Immunohistochemistry is an important tool in the diagnosis of MCC. The tumor cells have epithelial and neural properties because coexpression of cytokeratin filaments and neurofilaments can be observed. Immunohistochemical study results are positive for neuroendocrine markers (chromogranin, synaptophysin, neurospecific enolase and neurofilaments), low molecular weight cytokeratins and membrane epithelial antigen5. Especially, perinuclear dot-like staining for cytokeratin 20 is a characteristic finding.
MCC in general has a poor prognosis with a median survival of 33 to 47 months and has a high incidence of locoregional recurrence and systemic spread6. Hematogenous and/or distant lymphatic metastases ultimately occur in one-third of patients and most frequently involve liver, bone, brain, lung and skin, although metastasis to nearly every organ has been reported. Poor prognostic factors are: occurrence in patients aged over 55 years, location on the head and neck region, size of more than 2cm, the presence of immunosuppression, advanced stage of disease, more than 10 mitotic figures per single high-power field, small cell size, angiolymphatic invasion, and immunoreactivity for CD4478. Local recurrence is a major risk factor for further spreading and distant metastases, and worsens the prognosis4. Attempts to reduce the rate of loco-regional metastases might also be capable of improving the prognosis.
Treatment modalities for MCC are somewhat controversial but stage specific guidelines are helpful in directing optimal treatment. Current therapeutic guidelines suggest that MCC should be treated with locally aggressive methods including complete surgical excision and post-operative radiotherapy, probably including the locoregional lymph nodes9.
Surgical excision with wide clinical margin (2~3cm) is the mainstay of treatment for MCC without distant metastasis (e.g. stage I, II disease)5. However, MCC usually presents on head and neck areas where conservation of vital organs is important, so some clinicians proposed Mohs micrographic surgery with intraoperative evaluation of the peripheral and deep margins as the preferred surgical approach in locations where sparing healthy tissue is important5. Some studies also suggested that local recurrence rate with Mohs micrographic surgery was lower than with wide excision because of thorough histologic evaluation of the margin4. But, the true benefit of Mohs micrographic surgery remains unclear.
Evaluation and management of lymph node metastasis should be performed in all patients with MCC, because lymph node metastasis develops in up to 75% of patients4. But, Routine performance of elective lymph node dissection (ELND) is currently considered controversial because of potential morbidity, without proven survival benefit. Many recent studies suggest that, as alternative method, sentinel lymph node (SLN) mapping and biopsy could improve the ability to detect subclinical nodal metastases, sparing those patients with negative nodes the morbidity of lymph node dissection. SLN mapping and biopsy could provide a more accurate prognostic information for patients510. Sheela G et al. recently suggested that SLN biopsy should be routinely included in the evaluation of patients with MCC11.
The addition of post-operative irradiation of 45 to 60 Gy to the primary site and draining lymph node basins for 4 to 6 weeks has been found to decrease local recurrence5. In a series by Gillenwater et al., adjuvant radiotherapy reduced local recurrence rate from 59% to 27%12. Vennes MJ et al. also reported the addition of adjuvant radiotherapy showed a significant benefit in median disease-free survival, although there remained no benefit in overall survival13.
In advanced cases with distant metastasis, chemotherapy has been mainly used for palliative purposes. Because of morphologic and immunohistologic similarity of MCC to small cell lung cancer, chemotherapy regimens employed in small cell lung cancer may provide a useful guide4. But the impact of chemotherapy on survival has not been determined.
In conclusion, MCC is a rare but aggressive carcinoma. There is ongoing debate regarding the optimal treatment of this disease. However, recent evidence suggests that patients treated with surgery and adjuvant locoregional radiotherapy experience a better disease-free survival compared with those treated with surgery alone. We consider these aggressive treatments to have been beneficial for our patient. But, we think that regular follow-up and close observation for disease recurrence should be required for a longer period to confirm successful treatment.
Figures and Tables
References
1. Pfeifer T, Weinberg H, Brady M. Lymphatic mapping for Merkel cell carcinoma. J Am Acad Dermatol. 1997; 37:650–651.

2. Veness MJ. Merkel cell carcinoma (primary cutaneous neuroendocrine carcinoma): an overview on management. Australas J Dermatol. 2006; 47:160–165.

3. Chiarelli TG, Grant-Keis JM, Sporn JR, Rezuke WN, Whalen JD. Unusual presentation of a Merkel cell carcinoma. J Am Acad Dermatol. 2000; 42:366–370.

4. Boyse K, Foley EH, Bradley V, Scarborough D. Merkel cell carcinoma: a case report with treatment summary and updates. Cutis. 2004; 74:350–356.
6. Savage P, Constenla D, Fisher C, Thomas JM, Gore ME. The natural history and management of Merkel cell carcinoma of the skin: a review of 22 patients treated at the Royal Marsden Hospital. Clin Oncol. 1997; 9:164–167.

7. Urbatsch A, Sams WM Jr, Urist MM, Sturdivant R. Merkel cell carcinoma occurring in renal transplant patients. J Am Acad Dermatol. 1999; 41:289–291.

8. LeBoit PE, Burg G, Weedon D, Sarasin A. World Health Organization Classification of Tumours. Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press;2006.
9. Lawenda BD, Thiringer JK, Foss RD, Johnstone PA. Merkel cell carcinoma arising in the head and neck: optimizing therapy. Am J Clin Oncol. 2001; 24:35–42.
10. Mehrany K, Otley CC, Weenig RH, Phillips PK, Roenigk RK, Nguyen TH. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg. 2002; 28:113–117.

11. Gupta SG, Wang LC, Peñas PF, Gellenthin M, Lee SJ, Nghiem P. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006; 142:685–690.
12. Gillenwater AM, Hessel AC, Morrison WH, Burgess MA, Silva EG, Roberts D, et al. Merkel cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2001; 127:149–154.

13. Veness MJ, Perera L, McCourt J, Shannon J, Hughes TM, Morgan GJ, et al. Merkel cell carcinoma:improved outcome with adjuvant radiotherapy. ANZ J Surg. 2005; 75:275–281.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download