Abstract
Background
Angiogenesis is crucial for wound healing and exogenous supplements of the angiogenic growth factors have been known to promote cutaneous wound healing. Angiopoietin (Ang) 1 is a recently discovered angiogenic factor and there have been few studies of its effect on cutaneous wound healing.
Methods
Cartilage oligomeric matrix protein (COMP)-Ang 1 (Ade-COMP-Ang 1)- was intravenously injected to rats two days before surgery creating full-thickness wounds. The clinical wound healing rate and the number of vessels in the skin samples were evaluated on days 3, 7 and 14 post operation.
Results
At post-operation day 3, 7 and 14, the clinical wound healing rate was 38.3%, 59.4% and 92.1%, respectively, in the Ade-COMP-Ang 1-treated group, compared with 20.5%, 47.5% and 87.3%, respectively, in the Ade-LacZ-treated group. There were significant differences in the results of day 3 and day 7 between two groups (p<0.05). Histopathologically, the number of the vessels of the Ade-COMP-Ang 1-treated group was 73.7, 94.1 and 62.7 at day 3, 7 and 14, compared with that of the Ade-LacZ-treated group, 53.5, 83.9, and 56.9. The differences in the results of the two groups were statistically significant (p<0.05).
The closure of cutaneous wounds involves events such as hemorrhage, inflammation, re-epithelialization, granulation tissue formation, and the late remodeling phase of tissue repair. The early and acute phases of repair involve macrophage accumulation, fibroblast ingrowth, matrix deposition, and angiogenesis. These events are triggered by a complex mixture of cytokines and growth factors that are released at the site of injury1.
Angiogenesis is the sprouting of new blood vessels from a pre-existing network, and this process is central to the formation of granulation tissue because the ingrowth of newly formed vessels is needed to ensure the supply of oxygen, nutrients and inflammatory cells to the regenerating tissue12. Previous studies have shown that delayed wound healing such as diabetic ulcers, chronic venous ulcers, pressure ulcers, and cutaneous wounds in the aged, are associated with decreased angiogenesis and altered levels of angiogenic growth factors including vascular endothelial growth factor (VEGF) and angiopoietin (Ang)345. Based on these results, "therapeutic angiogenesis", i.e. supplementation of such recombinant angiogenic growth factors as VEGF, erythropoietin, nerve growth factor, platelet-derived growth factor, fibroblast growth factor2 and granulocyte-machrophage colony stimulating factor, has been used both in animal models and in clinical trials for patients to enhance cutaneous wound healing678.
Ang was discovered as a ligands for the tyrosine kinase with immunoglobulin and epidermal growth factor homology (Tie) 2 that was selectively expressed on the vascular endothelium. There are four definitive members of the Ang family, Ang 1, 2, 3 and 4. Although the functions of Ang and Tie have not been well established, Ang is thought to be involved in the proliferation, maturation, stabilization and remodeling of vessels19. Therefore, Ang 1 could be one of the promising candidate growth factors for therapeutic angiogenesis.
Large-scale production of recombinant Ang 1 is hindered by the aggregation and insolubility of protein. The activity of the purified protein frequently varies. These difficulties are due to its unique structural characteristics. COMP-Ang 1 is a soluble, stable, and potent Ang 1 variant in which the N-terminal portion of Ang 1 is replaced with the short coiled-coil domain of cartilage oligometric matrix protein (COMP). COMP-Ang 1 is more potent than native Ang 1 in phosphorylating Tie 2 and Akt in primary cultured endothelial cells and in angiogenesis in vivo1011.
In this study, we investigated the potential benefits of the therapy with adeno-associated systemic COMP-Ang 1 on cutaneous wound healing. The results of the study show that systemic COMP-Ang 1 treatment enhances cutaneous wound healing in vivo by promoting angiogenesis.
Recombinant adenoviruses that expressed COMPAng 1 or LacZ were constructed using the pAdEasy vector system (Ade-COMP-Ang 1 and Ade-LacZ) (Ade-COMP-Ang 1 and Ade-LacZ were generous gifts from Prof. Gou Young Koh at Korea Advanced Institute of Science and Technology, Daejeon, Korea).
Eighteen male Sprague-Dawley rats (200~250 g in weight) were housed two per cage, maintained under controlled environmental conditions (12 hlight to dark cycle, temperature approximately 23℃) and provided with standard laboratory food and water ad libitum. All the animal procedures were in accordance with the declaration of Helsinki and the guidelines for the care and use of laboratory animals. The animal care and experimental procedures were performed under approval from the Animal Care Committees of the Catholic University of Korea. For the adeno-viral treatment, 109 p.f.u. of Ade-COMP-Ang 1 and Ade-LacZ diluted in 50 µL of sterile 0.9% NaCl was injected intravenously into nine rats, through the tail vein two days before surgery.
General anesthesia with ketamine (80 mg/kg body weight)/xylazine (10 mg/kg body weight) was achieved by intraperitoneal injection. The hair on the back of each rat was shaved and the back skin was subsequently wiped with 70% ethanol. Three full-thickness wounds (1 cm in diameter, 2~3 cm apart to minimize the tension to adjacent wounds) were made on the back of each rat by excising the skin. The wounds were allowed to form scabs.
To compare the rate of wound healing between the Ade-COMP-Ang 1- and Ade-LacZ-treated groups, the animals were photographed on days 0, 3, 7 and 14 after surgery. In addition, the wounds were copied exactly onto transparent film with an indicated standard unit. The photographs and transparent films were digitally processed and the areas of the wounds were calculated using the KS300 system (Zeiss, Jena, Germany). For each sample, the rate of the healing process was measured as a percentage of the area of the wound at each time-point compared to the area of the wound at day 0.
Three wounds on the back of each rat were sequentially removed from the site at days 3, 7 and 14 after surgery. Each specimen was divided into two equal parts. One half of the specimens were fixed in 4% paraformaldehyde, washed in tap water, dehydrated in a series of graded ethanol solutions, cleared in xylene, and then embedded in paraffin for light microscopic examination. The sections (4 µm thickness) were then mounted on glass slides, hydrated with distilled water, and subjected to hematoxylin-eosin staining (Vector Laboratories, Burlingame, CA, USA)6.
The other half of each specimen was embedded in OCT compound (Sakura, Tokyo, Japan) for immunostaining. The skin specimens were prepared for immunohistochemical staining as previously described6. Briefly, five-micrometer cryostat sections were cut on a cryostat microtome (Leica Microsystems AG, Wetzlar, Germany), and then fixed in acetone for 15 min. After three washes in phosphate buffered saline (PBS), the sections were incubated for 10 minutes in methanol with 0.3% hydrogen peroxide to block the endogenous peroxidase activity. Nonspecific antibody binding was blocked by incubating the sections with 10% normal donkey serum (Jackson Immuno Research Laboratories, West Grove, PA, USA) for 60 min. The sections were incubated with mouse anti-rat PECAM-1/CD31 monoclonal antibodies (1:100 in PBS, Cemicon, CA, USA) overnight at 4℃. Subsequently, the tissue sections were incubated with secondary antibodies (1:300 in PBS, Jackson Immuno Research Laboratories) for 60 min. As controls, the same skin specimens were incubated with an isotype-matched antibody. As a negative control, skin samples were incubated with PBS without the primary antibodies. The color reaction of the treated tissue was carried out using the substrate 3-amino-9-ethylcarbazole (Vector Laboratories) for 10 min. The skin sections were also counterstained with hematoxylin (Vector Laboratories) for 1 to 2 min.
The number of blood vessels with definite lumens or with red blood cells in it was counted under a light microscope under the high power view (×200) by two independent dermatologists who were without knowledge of the previous treatment, and then the mean number and standard deviation of vessels in the Ade-COMP-Ang 1-treated and Ade-LacZ-treated groups were calculated.
The data were analyzed by two-way repeated measures analysis of variance (ANOVA) tests, two samples t-tests and paired t-tests were performed for statistical analysis. The data were analyzed by the statistical program SPSS for Windows 10.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as p<0.05.
Analysis of the clinical wound healing rate in Ade-COMP-Ang 1-treated and Ade-LacZ-treated groups was performed via digital processing at 0, 3, 7 and 14 days after the wounding. The Ade-COMP-Ang 1-treated group showed 39.5%, 59.3%, and 91.8% reduction of the wound area at day 3, 7, and 14, respectively. On the other hand, the results in the Ade-LacZ-treated group revealed 20.4%, 46.2% and 89.0% reduction of the wound area, respectively. The differences in the results between the two groups at day 3 and 7 were statistically significant (p<0.05), however, the differences in the results at day 14 failed to show a statistical significance (Fig. 1).
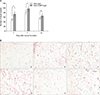
In order to investigate whether the Ade-COMP-Ang 1-induced-acceleration of wound healing was associated with an angiogenic effect, the number of vessels of the samples was evaluated by two independent investigators under a high power view (×200) at 3, 7, and 14 days after wound surgery. The mean vessel counts per specimen were averaged to give an overall mean vessel count per high-power field for each wound section. The number of blood vessels of the Ade-COMP-Ang 1-treated group was 73.7, 94.1, and 62.7 at day 3, 7, and 14, compared with the Ade-LacZ-treated group, i.e. those number of blood vessels was 53.5, 83.9 and 56.9, respectively. There were significant differences in the results at day 3, 7 and 14 between the two groups (p<0.05) (Fig. 2).
The angiogenic effect of Ang 1 has been reported in previous studies. Ang 1 and Tie 2-deficient mice had similar phenotypes that were characterized by embryonic lethality with severe vascular remodeling defects, insufficient vessel stabilization and perturbed vascular maturation1213. Promoted angiogenesis has been reported on for transgenic mice that overexpress Ang 1 in the skin14. In addition, there have been reports that exogenous and localized Ang 1 treatment with naked DNA-mediated or adenovirus-mediated Ang 1 enhanced angiogenesis in the gastric ulcer model or the skin flap model1516. In this study, we wanted to verify whether a systemic adenovirus-associated COMP-Ang 1 treatment can induce angiogenesis in a cutaneous wound healing model. Our results demonstrated that angiogenesis in the COMP-Ang 1 treated group was significantly increased at day 3, 7 and 14, compared with the control group. Although we did not confirm the expression of COMP-Ang 1 either in the plasma or in the cutaneous wound after systemic injection, the level of systemic circulating COMP-Ang 1 is known to be increased as early as 12 hours after Ade-COMP-Ang 1 injection, peaking at 1 to 2 weeks and gradually declining thereafter. The levels return to the control levels at 6 weeks after systemic treatment in the murine model10. Therefore, we could infer that the enhanced angiogenesis in the COMP-Ang 1 treated group is attributable to the increased level of COMP-Ang 1 in the plasma.
The results of this study showed that adenovirus mediated COMP-Ang 1 therapy significantly enhanced the reduction of wound size at day 3 and 7 after wound surgery; however, there was no significant difference at day 14. These data coincided with previous reports showing the difference of the reduction of the wound size did not reach statistical significance after day 7 or 9 after wounding, in an adenovirus-mediated VEGF treatment model or a cultured autologous fibroblast transplant model, respectively617.
A recent explosion of newly discovered growth factors that act on the vascular endothelium has coincided with the application of powerful new genetic approaches to the problem of vascular development. The vascular endothelium-specific growth factors include five members of the VEGF family, four members of the Ang family, and at least one member of the large ephrin family1. In addition, many other growth factors that are not vascular endothelium-specific are also required for blood vessel formation, and these include the members of the platelet-derived growth factor families or the transforming growth factor families. However, the critical roles on many other systems of the latter limit their clinical application. VEGF has been the most commonly studied and used growth factor for promoting wound healing. The previous studies have shown that VEGF is a more potent growth factor for endothelial proliferation than is Ang 115. However, continual overexpression of VEGF has been found to result in hemangioma-type tumors18, indicating that the VEGF expression or effective dose must be tightly regulated and that the expression must transiently occur only at the early stage of wound healing. Furthermore, the microvessels that develop from the continual overexpression of VEGF were found to be disorganized, sinusoidal and leaky because of VEFG's additional function as a vascular permeability factor19. In contrast, Ang 1 induces maturation, stabilization and remodeling of vessels and overexpression of Ang 1 does not result in vascular leakage202122. Therefore, Ang 1 is thought to have a wider therapeutic window and it could be a safer modality for clinical use.
As far as treatment with growth factors is concerned, naive protein treatment in the clinical setting is limited by several factors, such as their short half-lives, their inactivation by wound proteases, their poor bioavailability from the utilized vehicles and consequently the need for daily applications and high initial doses that might become toxic23. An alternative approach that might overcome most of these problems is the delivery of growth factor-encoding genes. Gene therapy could overcome the short-comings of direct application of the growth factor and so promote continual production and release of the growth factor within the blood.
In conclusion, the results of this study suggest that Ade-COMP-Ang 1 gene therapy significantly accelerates acute cutaneous wound healing by promoting angiogenesis at the site of injury. Further study using Ade-COMP-Ang 1 gene therapy in chronic wounds will be needed in the near future.
Figures and Tables
Fig. 1
(A) The effect of Ade-COMP-Ang 1 on clinical wound healing. The treatment of Ade-COMP-Ang 1 showed 39.5%, 59.3% and 91.8% reduction of wound size at day 3, 7 and 14, compared with 20.4%, 46.2% and 89.0%, in the Ade-LacZ treated group, respectively. The results of day 3 and 7 between the Ade-COMP-Ang 1 treated group and the Ade-LacZ treated group were statistically significant (*p<0.05). (B) A representative example of the cutaneous wound healing of the Ade-COMP-Ang 1-treated and Ade-LacZ treated groups at day 0, 3, 7 and 14. The wound healing rate was accelerated in the Ade-COMP-Ang 1-treated group, compared with Ade-LacZ-treated group. Ade-LacZ-treated group: a (day 0), b (day 3), c (day 7), d (day 14 after wound surgery), Ade-COMP-Ang 1-treated group: e (day 0), f (day 3), g (day 7), h (day 14 after wound surgery).
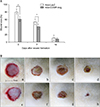
Fig. 2
(A) The effect of Ade-COMP-Ang 1 on angiogenesis. Under the high power field view (×200), the number of blood vessels of the Ade-COMP-Ang 1-treated group was 73.7, 94.1 and 62.7 at day 3, 7 and 14, respectively, compared with that of the Ade-LacZ treated group, i.e., 53.5, 83.9, and 56.9, respectively. The results of day 3, 7 and 14 between the Ade-COMP-Ang 1 treated group and the Ade-LacZ treated group were statistically significant (*p<0.05). (B) The blood vessel formation of the Ade-COMP-Ang 1 treated and Ade-LacZ treated groups at day 3, 7 and 14 (PECAM, ×200). The number of blood vessels were increased in the the Ade-COMP-Ang 1-treated group, compared with Ade-LacZ-treated group. Ade-LacZ-treated group: a (day 3), c (day 7), e (day 14 after wound surgery), Ade-COMP-Ang 1-treated group: b (day 3), d (day 7), f (day 14 after wound surgery).
References
1. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000; 407:242–248.

2. Kämpfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and 2 and the Tie-1 and 2 receptor tyrosine kinases during cutaneous wound healing: A comparative study of normal and impaired repair. Lab Invest. 2001; 81:361–373.

3. Colwell AS, Beanes SR, Soo C, Dang C, Ting K, Longaker MT, et al. Increased angiogenesis and expression of vascular endothelial growth factor during scarless repair. Plast Reconstr Surg. 2005; 115:204–212.
4. Yao F, Visovatti S, Johnson CS, Chen M, Slama J, Wenger A, et al. Age and growth factors in porcine full-thickness wound healing. Wound Repair Regen. 2001; 9:371–377.

5. Drinkwater SL, Burnard KG, Ding R, Smith A. Increased but ineffectual angiogenic drive in nonhealing venous leg ulcers. J Vasc Surg. 2003; 38:1106–1112.

6. Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, et al. Adenovirus- mediated VEGF165 gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Therapy. 2002; 9:1271–1277.

7. Galeano M, Altavilla D, Cucinotta D, Russo GT, Calò M, Bitto A, et al. Recombinant human erythropoietin stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetes. 2004; 53:2509–2517.

8. Cianfarani F, Tommasi R, Failla CM, Viviano MT, Annessi G, Papi M, et al. Granulocyte/macrophage colony-stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed. Br J Dermatol. 2006; 154:34–41.

9. Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996; 87:1161–1169.

10. Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, et al. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. PNAS. 2004; 101:5547–5552.

11. Cho CH, Kammerer RA, Lee HJ, Yasunaga K, Kim KT, Choi HH, et al. Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004; 101:5553–5558.

12. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996; 87:1171–1180.

13. Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993; 90:9355–9358.

14. Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998; 282:468–471.

15. Jones MK, Kawanaka H, Baatar D, Szabó IL, Tsugawa K, Pai R, et al. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001; 121:1040–1047.

16. Jung H, Gurunluoglu R, Scharpf J, Siemionow M. Adenovirus-mediated angiopoietin-1 gene therapy enhances skin flap survival. Microsurgery. 2003; 23:374–380.

17. Kim GM, Lee WS, Hwang SJ, Kye YC, Kim HO, Kim SY. The study of wound healing using cultured autologous dermal fibroblast of guinea pig. Korean J Dermatol. 2005; 43:576–586.
18. Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000; 103:898–901.
19. Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000; 6:460–463.

20. Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999; 286:2511–2514.

21. Baffert F, Le T, Thurston G, McDonald DM. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am J Physiol Heart Circ Physiol. 2006; 290:H107–H118.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download