INTRODUCTION
Malignant eccrine poroma is a rare skin appendageal tumor occurring on the intraepidermal ductal portion of eccrine sweat glands. It was initially described as epidermotrophic eccrine carcinoma by Pinkus and Mehregan1 in 1963. In 1969, Mishima and Morioka2 used the term, eccrine porocarcinoma, subdividing the malignant form of benign eccrine poroma into poroacanthoma, poroepithelioma and porocarcinoma by a degree of differentiation. Although about 120 cases have been reported so far, the incidence and clinical aspects of malignant eccrine poroma have not yet been identified yet. Malignant eccrine poroma is known to develop on the lower limbs of the aged and sometimes metastasize to regional lymph nodes and internal organs, causing fatal consequences. In Korea, four cases have been reported: one case developed on the left buttock and metastasized to lymph nodes3; another developed on the right thigh4; another developed on the right scalp5; and the other developed on the antihelix6. However, only 5 cases of malignant eccrine poroma developing on the suprapubic area reported to date7.
CASE REPORT
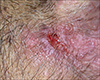
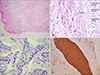
A 75-year-old man visited with complaints of an ulcerative tumor developing on the suprapubic area. The clinical manifestation was a slow-growing ulcerative nodule without any symptoms for 3 months. He had no specific past history or any physical findings such as enlarged lymph nodes. Physical examination showed a 2 × 2 cm2 nodule with an erythematous ulcerated surface on the suprapubic area (Fig. 1). Chest X-ray and the laboratory test results were normal. Deep biopsy was done on the ulcerating margin of the skin. Histopathologic findings showed marked acanthosis and a well-demarcated tumor area. Some tumor nests were connected to the epidermis and others freely floated without connection to the epidermis (Fig. 2A). When highly magnified, the nest was found to be a mixture of cells with small, relatively regular sized nuclei and cells with large, hyperchromatic atypical nuclei. Some cells had vacuoles in the cytoplasm (Fig. 2B). On immunohistochemistry, the nests showed positivity for PAS and EMA (Fig. 2C, D). Most of the tumor cells showed negativity for carcinoembryonic antigens, whereas tumors forming the structures of vessels showed positivity. They showed negativity for S-100 protein and positivity for cytokeratin. We referred him to the department of urology for surgical excision. There has been no recurrence six months after the operation.
DISCUSSION
Malignant eccrine poroma (eccrine porocarcinoma) is a rare skin appendage tumor occurring on the intraepidermal ductal portion of eccrine sweat glands, first described by Pinkus and Mehregan1 in 1963. Five cases3456 have been reported in Korean literature since the initial two cases of immunohistologic stain findings were reported by Sung et al8 in 1992. Malignant eccrine poroma occurs mainly in the aged. It can develop on any part of the body but is typically seen on lower extremities7. Approximately 50% of all tumors develop on the lower extremities, among them the most common tumor sites are legs (30%) and feet (18%). Studying 120 cases of malignant eccrine poroma revealed that the leg is the most vulnerable site, followed by the foot, face, thigh and arm7. Apparently the site of malignant eccrine poroma has nothing to do with the density of sweat glands. Although the palm and sole contain the highest densities of eccrine sweat glands, only one case reportedly involved the finger and palm and only seven cases involved the sole7. In this case, the tumor developed on the suprapubic area, which is a rare location in that only 5 such cases have been reported in English literature. Clinically, the disease displays visual symptoms like verrucous plaques and ulcerative nodules. If it is accompanied with multiple lesions, it suggests local recurrences or metastases to internal organs79.
In cases where malignant eccrine poroma develops on benign eccrine poroma that has existed for a long time, anaplastic cells are to be discovered. They appear to be pathologically benign, carrying hyperchromic nuclei in large and irregular shapes and lying next to the basophilic uniform columnar cells of eccrine poroma. Other characteristic features include hyperkeratosis, acanthosis and cloned tumor cell nests that are different in shape and clearly separated from the surrounding squamous cells, which is called Jadassohn phenomenon. Care should be taken to differentiate it from other dermatological diseases such as benign eccrine poroma, seborrheic keratosis and Bowen's disease7. This case also includes marked clonal epidermal tumor cell nests and the infiltration of tumor cell nests into the dermis. Tumor cells have large nuclei with multiple atypical mitoses and cytoplasmic vacuoles. They are considered as an undifferentiated ductal portions of the eccrine sweat gland in the tumor cells. One of the most important findings in diagnosing malignant eccrine poroma is the ductal structures and cytoplasmic vacuoles surrounded by eosinophilic cuticles, which indicates eccrine differentiation7.
On immunohistochemistry, EMA and CEA are known to be useful to distinguish between normal eccrine glands and tumors arising on eccrine glands7. S-100 protein is seen on secretory cells of normal eccrine glands and myoepithelial cells, but not in cells of the ductal portion of eccrine sweat glands or tumors arising on eccrine glands8. Malignant eccrine poroma arising on the intraepidermal ductal portion of eccrine sweat glands shows positivity for EMA and negativity for S-100 protein. Most of tumor cells show negativity for CEA but tumor cells forming vessel structures show positivity for CEA8.
Malignant eccrine poroma arises on the intraepidermal ductal portion of eccrine sweat glands. Tumor cells grow horizontally from epidermis to surrounding parts or vertically from epidermis into dermis and even into subcutaneous tissue710. Tumors can also cause lymphangitis carcinomatosa in the event that they infiltrate into the lymph nodes of dermis110, which clinically causes lymphedema in which the superficial lymphatics are blocked by tumor cells that act like a plug.
Malignant eccrine poroma recurs in patients who have had their tumor surgically removed and spreads to nearby lymph nodes after 1 to 6 months in 20% of all patients. Thus, because the tumor is likely to recur regionally, it is important to extensively excise and completely remove the area around the tumor while closely looking into the margin of the tumor through a microscope. It is also necessary to do prophylactic regional lymph node dissection in case of regional lymphadenopahty7. Once the disease recurs, however, most patients die of metastases after they don't respond to chemotherapy or radiotherapy due to incorrect diagnosis7. In conclusion, we clinically and pathologically experienced one case of malignant eccrine poroma developing on suprapubic area, which is considered a very rare case, and report it along with immunohistologic findings.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download