Abstract
Renal cell carcinoma (RCC) is well known for its frequent metastasis and particularly to the lungs, liver, bones and brain, but metastasis to the skin is rare. We report here on a case of metastatic RCC in a 73-year-old man who presented with a 1.5 cm sized, moist, beefy-red and exophytic nodule on the scalp. The lesion had grown rapidly for 2 months and it clinically mimicked granuloma pyogenicum. A skin biopsy revealed a solid mass composed of clear cells with clear cytoplasm and oval hyperchromatic nuclei, and they were arranged in an alveolar pattern. As skin metastasis from renal cell carcinoma signals widespread systemic metastasis and a poor prognosis, clinicians should conduct a careful inspection of the skin of a patient with RCC and they should also have a high index of suspicion for finding a primary internal organ malignancy in the RCC patients who present with a skin lesion.
Renal cell carcinoma (RCC) accounts for 2~3% of all adult malignancies and the incidence of cutaneous metastasis from this tumor is 2.8~6.8%1. Metastasis from renal cell carcinoma to the internal organs such the lung, lymph nodes and bone is well known, but there are far fewer reports of metastasis to the skin.
Cutaneous metastasis of RCC is extremely uncommon with only a handful of reports in the literature. We report here on a case of renal cell carcinoma that presented as a solitary cutaneous lesion that mimicked granuloma pyogenicum on the scalp.
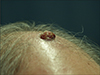
A 73-year-old man presented with a nodule on his vertex. He had noted this lesion 2 months previously, and it had rapidly increased in size. Examination of the skin revealed a 1.5 cm sized, moist, beefy-red and exophytic nodule, which clinically mimicked granuloma pyogenicum (Fig. 1). Four years previously, he had visited our hospital where he underwent abdominal computed tomography, and this showed a mass on the right kidney. A radical nephrectomy was performed at that time and this revealed an 8 cm sized intraparenchymal mass. The lymph nodes were negative for metastatic disease and the patient had negative findings on the chest radiographs and computed tomography. According to the TNM staging system, he had stage II disease and no adjunctive therapy was required. However, 5 months previously, a CT scan of the chest and abdomen showed evidence of a nodule on the right lung. Percutaneous needle aspiration biopsy on the right lower lobe revealed atypical clear cells and this suggested that RCC had metastasized to the lung. For further evaluation, an ultrasound sonography-guided lung biopsy was recommended, but he refused.
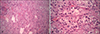
A skin biopsy revealed a solid mass composed of clear cells with clear cytoplasm and oval hyperchromatic nuclei (Fig. 2). The nests of clear cells were arranged in an alveolar pattern. The cytoplasm of the tumor cells stained positive for epithelial membrane antigen (EMA) and cytokeratin (Fig. 3), but the staining for carcinoembryogenic antigen (CEA) was negative. The histologic diagnosis was metastatic RCC. It has now been two and a half years since the diagnosis, and the patient is alive and doing well after mass excision.
RCC has been well described concerning its frequency to metastasize, and this occurs in 25~30% of RCC patients at the time of diagnosis2. The incidence of cutaneous metastasis is 2.8~6.8%, with the more common sites being the lung, lymph nodes and bone1. The most common sites of cutaneous metastases of RCC are the head and neck, although cutaneous metastases may occur on the trunk or extremities.
Presentation with the classic triad of RCC, including flank pain, hematuria and a palpable abdominal mass, is uncommon. Most patients present with only one of the above symptoms or an incidental diagnosis of RCC was made as a result of radiological imaging that was done for another reason. Some patients may present with symptoms from secondary metastases.
The clinical manifestation of cutaneous metastases often appear as well-circumscribed, cutaneous nodules that are either flesh-colored, violaceous, or blue, although unusual manifestations such as a cutaneous horn have been reported3. These nodules may be solitary or wide-spread.
The histologic features are usually those of a clear-cell adenocarcinoma4. The tumor cells show oval nuclei with abundant, clear cytoplasm and the cells are often are in a glandular configuration. The stroma is vascular, and there are frequent extravasated red blood cells. Intracytoplasmic glycogen is uniformly present as demonstrated by PAS staining and diastase-labile intracytoplasmic material is also present.
RCC must be differentiated from other clear cell carcinomas that occasionally metastasize to the skin. These carcinomas include primary tumors of the lung, liver, ovary, endometrium, cervix and vagina5. Primary tumors of the skin that must be differentiated from RCC, and these primary tumors include eccrine acrospiroma and sebaceous tumors4. In contrast to RCC, eccrine acrospiroma is usually multilobular and it has prominent ductal structures, but it is not associated with prominent vascularity, hemorrhage or hemosiderin deposition. Sebaceous tumors are not as vascular as renal tumors and they have fine vacuolated cytoplasm.
Hematogenous dissemination from a renal neoplasm can occur by three routes6. First, the most important route is via the renal vein to the vena cava to the right atrium and lung. Massive invasion of the renal vein or vena cava by a neoplastic thrombus is a distinguishing characteristic of RCC. Second, invasion of the spermatic vein permits reverse metastasis to the pelvic organs. Third, invasion of the vertebral veins with their low pressure and inversion of flow leads to preferential localization in the vertebral column, thyroid and central nervous system. The last route may explain head and neck metastases.
More than half of all cases with cutaneous metastasis from RCC have undergone nephrectomy within 2 years prior to the manifestation of the cutaneous lesions, although in some cases the skin involvement appears after a long interval7. One case reported in the literature had undergone radical nephrectomy for RCC 15 years before manifesting skin metastasis8. In spite of the long interval between nephrectomy and the diagnosis of skin metastasis, the prognosis of these patients is poor because by the time the metastasis is discovered, the underlying disease has already become widespread9. Thus, a thorough search is recommended when a patient presents with skin metastasis after radical nephrectomy. The mean survival time after detection of cutaneous metastasis is approximately 7 months10. Our case presented with skin and lung metastases 4 years after he underwent nephrectomy.
In summary, skin metastasis from RCC is uncommon and this is regarded as a late manifestation that implies a poor prognosis. Therefore, it is important to carry out detailed examinations that include the skin of patients with RCC for several years after nephrectomy.
We report here on a case of metastatic RCC on the vertex, and this tumor presented as a rapidly growing solitary tumor that mimicked granuloma pyogenicum.
Figures and Tables
References
1. Choi JY, Han KS, Bang HD, Kim KH, Kim KJ, Kim JM. A case of renal cell carcinoma metastatic to the scalp. Korean J Dermatol. 2001; 39:711–713.
2. Jeong E, Lee CN, Park HJ, Oh ST, Lee JY, Cho BK. A case of acrometastasis in renal cell carcinoma. Korean J Dermatol. 2004; 42:472–477.
4. Johnson WC. Metastatic carcinoma of the skin: incidence and dissemination. In : Elder DE, Elenitsas R, Johnson BL, Murphy GF, editors. Lever's histopathology of the skin. 9th ed. Philadelphia: Lippincott Williams & Wilkins;2004. p. 1149–1157.
6. Snow S, Madjar D, Reizner G, Mac KE, Bentz M. Renal cell carcinoma metastatic to the scalp: case report and review of the literature. Dermatol Surg. 2001; 27:192–194.

7. Haruki T, Takahashi S, Morohashi M, Maruyama T, Ida M. Cutaneous metastasis of renal cell carcinoma: an electron microscopic study. J Dermatol. 1991; 18:218–224.

8. Hongo F, Saitoh M, Tsuruyama K, Shimoo K, Yamamura Y. Renal cell carcinoma with late skin metastasis: a case report. Hinyokika Kiyo. 1999; 45:415–417.
9. Koga S, Tsuda S, Nishikido M, Matsuya F, Saito Y, Kanetake H. Renal cell carcinoma metastatic to the skin. Anticancer Res. 2000; 20:1939–1940.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download