Abstract
The nevoid basal cell carcinoma syndrome, or Gorlin-Goltz syndrome, is an autosomal dominant multiple system disorder with high penetrance and variable expressions, although it can also arise spontaneously. The diagnostic criteria for nevoid basal cell carcinoma syndrome include multiple basal cell carcinomas, palmoplantar pits, multiple odontogenic keratocysts, skeletal anomalies, positive family history, ectopic calcification and neurological anomalies. We report a brother and sister who were both diagnosed with nevoid basal cell carcinoma syndrome.
The nevoid basal cell carcinoma syndrome (NBCCS), or Gorlin-Goltz syndrome, is an autosomal dominant multiple system disorder with high penetrance and variable expressions1. However, 60% of patients with NBCCS are sporadic cases. It has an estimated prevalence of 1 in 60,000 with equal distributions among males and females2. The well-defined diagnostic criteria include cutaneous anomalies, dento-facial anomalies, skeletal anomalies, positive family history, neurological anomalies, genitourinary anomalies and other congenital malformations. For cutaneous anomalies, basal cell carcinoma (BCC) may arise at any site of the body, although the periorbital area, eyelids, nose, upper lip and malar region are the most commonly affected areas. We report a brother and sister who were both diagnosed with nevoid basal cell carcinoma syndrome.
An 11-year-old male was referred to our department for the evaluation of multiple miliary sized pigmented macules on the palm and sole that had increased in number over several years. He had an operation for inguinal hernia at 3 years of age, but no other medical problems. One month prior to his referral, multiple jaw cysts in the maxilla were identified by a dentist (Fig. 1). The cysts were treated and decompressed by incisional biopsy. The cysts were diagnosed as odontogenic keratocysts. There was no family history of skin cancer or related syndromes. His 8-year-old sister also had a cyst in the right mandible, which was diagnosed as an odontogenic keratocyst.
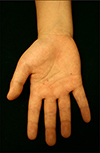
Physical examination revealed a slightly enlarged head circumference, ocular hypertelorism and multiple miliary sized pigmented macules on the palm (Fig. 2) and sole. Several skin biopsies were performed of the pigmented macules of the left palm for pathologic diagnosis.
Histopathologically, the lesions showed well-circumscribed basaloid neoplasms comprised of basaloid neoplastic cells that had large oval or elongated basophilic nuclei with relatively little cytoplasms. These cells were arranged in the upper dermis in an anastomosing fashion, with scant stroma and peritumoral lacunae (Fig. 3). There were variable degrees of cytologic atypia and mitotic activities. In basaloid follicular hamartoma, strands and cords of small, basaloid cells emanate from the infundibular portion of the hair follicle and there is no significant clefting between the tumor and stroma and no mitotic activity that differentiates it from BCC. Thus, the specimens were consistent with BCC.
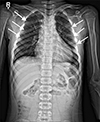
Radiographic findings included straightening of C-spine lordosis, mild intervertebral disc space narrowing at C4-5, mild shortening of the 4th and 5th metacarpals of both hands and bilateral deformities of the 3rd, 4th and 5th ribs (Fig. 4). On the basis of these clinical and histological findings, the patient was diagnosed with NBCCS. All lesions that suggested basal cell carcinoma were removed from the palmar surface by punch excision and a CO2 laser.
An 8-year-old female with no significant past medical history came to the Department of Dermatology for evaluation of NBCCS. She had a brother who was diagnosed with NBCCS. Physical examination revealed a slightly enlarged head circumference, mild ocular hypertelorism and no other cutaneous abnormalities. We referred her to the Department of Obstetrics and Gynecology for evaluation of genitourinary anomalies where a 1×0.5×0.5 cm sized vulvar tumor was detected. It was diagnosed as a fibro-epithelial polyp by excisional biopsy. A radiographic examination showed a welldefined, radiolucent cystic lesion that was located in the right mandibular body. It was diagnosed as an odontogenic keratocyst by incisional biopsy. However, we found no any other skeletal abnormalities.
NBCCS was first described by Jarish in 1894 for a patient with multiple basal cell carcinomas,scoliosis and learning difficulties2. However, the condition as a syndrome comprising the principal triad of multiple basal cell nevi, jaw keratocysts and skeletal anomalies was initially defined by Gorlin and Goltz3 in 1960. This disorder has an autosomal dominant pattern of inheritance. However, it can also arise spontaneously or have a variable phenotypic penetration.
NBCCS has been linked to germline mutations in the human homologue of the Drosophila segment polarity gene patched (PTCH)4. The mutated gene, PTCH, is a classic tumor suppressor gene that was mapped to chromosome band 9q22.3. The gene consists of 23 exons and 12 transmembranespanning domains that code for 2 large hydrophilic extracellular loops where Sonic Hedgehog ligand binding occurs5. In the hedgehog signaling network, mutations result in various phenotypes, including holoprosencephaly, NBCCS, Pallister-Hall syndrome, Greig cephalopolysyndactyly, Rubinstein-Taybi syndrome, isolated BCCs and desmoplastic medulloblastoma, among others. Soufir et al6 identified PTCH mutations in 12 of 17 patients showing the full complement of NBCCS criteria. However, 35~50% of cases are new germline mutations7. Indeed, nearly 60% of patients with NBCCS have no known affected family members.
The diagnostic criteria for NBCCS include variable abnormalities and a positive family history (Table 1)8. Odontogenic keratocysts are often the first sign of NBCCS and arise earlier in patients with the syndrome than in patients unaffected by the syndrome9. The most frequent skin lesions for NBCCS are BCCs. Epidemiological studies10 suggest that sunlight, and UVB radiation in particular, is a strong risk factor for the formation of BCC. BCC numbers may vary from only a few to thousands and range in size from 1 to 10 mm in diameter. The sites most often involved are the face, back and chest. Most show little growth. It is only after puberty that the BCCs can become aggressive and locally invasive 5.
BCCs become clinically manifest between puberty and 35 years of age. Histologically, BCCs in NBCCS cannot be differentiated from BCCs that are unrelated to NBCCS. Palmar and plantar pits that measure about 1~2 mm in diameter are asymmetrically present in 65% to 80% of cases11. The pits usually develop during the second decade, although BCCs rarely arise in these pits. However, a thorough relational study is lacking. To date, there has been no study regarding the prevalence of BCC arising from the palmar surface. Howell et al12 reported that epidermal rete ridges beneath the pits are found to be amassed with cells that resemble those of BCC. Thus, these pits represent formes frustes of BCC. In some patients, the pits actually show the presence of small BCCs at their base13.
Calcification of the falx cerebri, the most frequent radiologic feature, is found in 55% to 95% of NBCCS patients as opposed to, perhaps, 5% in the general population. Kimonis et al14 reported for 82 NBCCS patients that calcification of the falx cerebri was present in 79% older than 20 years and in 37% who were younger than 20 years. We could not find calcification of the falx cerebri in our cases. Other features of skeletal anomalies are fused, splayed or bifid ribs, spina bifida occulta of the cervical or thoracic vertebrae, polydactyly, syndactyly and shortening of the 4th and 5th metacarpals. Scoliosis and rib anomalies are commonly seen, but vertebral anomalies are not common. We found rib anomalies and shortening of the 4th and 5th metacarpals in one of our cases.
In the Korean literature, 16 cases with family histories of NBCCS have been reported (Table 2). In the field of dermatology, Joh et al15 reported 2 cases of NBCCS for a brother and sister, Tak et al16 reported 2 cases of NBCCS for sisters and Roh et al17 reported a case of NBCCS with a family history.
We report an additional 2 rare cases of NBCCS occurring in a Korean brother and sister. The older brother had odontogenic keratocysts, basal cell carcinoma developing on the palmoplantar surface, rib anomalies and shortened fourth and fifth metacarpals of both hands. The younger sister showed odontogenic cysts and a positive family history that satisfied the diagnostic criteria for NBCCS. Further evaluation and follow up, including genetic analysis, will be needed in both cases.
Figures and Tables
Fig. 3
Histological section of pigmented macules on the palmar surface shows a well-circumscribed neoplasm comprised of basaloid neoplastic cells arranged in an anastomosing fashion (H&E stain, ×100).
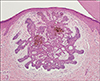
Table 1
Diagnostic criteria for Nevoid basal cell carcinoma syndrome taken from Crawford and Kobayashi8

References
1. Lo Muzio L, Nocini P, Nocini P, Pannone G, Consolo U, Procaccini M. Early diagnosis of nevoid basal cell carcinoma syndrome. J Am Dent Assoc. 1999; 130:669–674.

2. Gorlin RJ. Nevoid basal cell carcinoma (Gorlin) syndrome: unanswered issues. J Lab Clin Med. 1999; 134:551–552.

3. Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960; 262:908–912.

4. Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996; 85:841–851.

6. Soufir N, Gerard B, Portela M, Brice A, Liboutet M, Saiag P, et al. PTCH mutations and deletions in patients with typical nevoid basal cell carcinoma syndrome and in patients with a suspected genetic predisposition to basal cell carcinoma: a French study. Br J Cancer. 2006; 95:548–553.

7. Jih DM, Shapiro M, James WD, Levin M, Gelfand J, Williams PT, et al. Familial basaloid follicular hamartoma: lesional characterization and review of the literature. Am J Dermatopathol. 2003; 25:130–137.
8. Crawford KM, Kobayashi T. Nevoid basal cell carcinoma syndrome or multiple hereditary infundibulocystic basal cell carcinoma syndrome? J Am Acad Dermatol. 2004; 51:989–995.

9. Lo Muzio L, Nocini PF, Savoia A, Consolo U, Procaccini M, Zelante L, et al. Nevoid basal cell carcinoma syndrome. Clinical findings in 37 Italian affected individuals. Clin Genet. 1999; 55:34–40.

10. Gailani MR, Bale SJ, Leffell DJ, DiGiovanna JJ, Peck GL, Poliak S, et al. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell. 1992; 69:111–117.

11. Shanley S, Ratcliffe J, Hockey A, Haan E, Oley C, Ravine D, et al. Nevoid basal cell carcinoma syndrome: review of 118 affected individuals. Am J Med Genet. 1994; 50:282–290.

12. Howell JB, Freeman RG. Structure and significance of the pits with their tumors in the nevoid basal cell carcinoma syndrome. J Am Acad Dermatol. 1980; 2:224–238.

13. Holubar K, Matras H, Smalik AV. Multiple palmar basal cell epitheliomas in basal cell nevus syndrome. Arch Dermatol. 1970; 101:679–682.

14. Kimonis VE, Mehta SG, Digiovanna JJ, Bale SJ, Pastakia B. Radiological features in 82 patients with nevoid basal cell carcinoma (NBCC or Gorlin) syndrome. Genet Med. 2004; 6:495–502.

15. Joh GY, Kim KH, Nam JT, Kim YS, Lee BK. Two cases of nevoid basal cell carcinoma syndrome. Korean J Dermatol. 1992; 30:951–957.
16. Tak WJ, Shin BJ, Kim MN, Ro BI. A case of nevoid basal cell carcinoma syndrome with multiple, symmetrically distributed basal cell carcinomas. Korean J Dermatol. 2002; 40:682–685.
17. Roh BH, Park SJ, Lee SY, Lee JS, Whang KU. A case of nevoid basal cell carcinoma syndrome with meningioma and sclerosing stromal tumor of the ovary. Korean J Dermatol. 2006; 44:1030–1033.
18. Lee SC, Kim YG, Kim HJ. Basal cell nevus syndrome: case reports. J Kyung Hee Univ Med Cent. 1986; 2:404–414.
19. Lee SC, Kim YG, Ryu DM, Cho SK. Basal cell nevus syndrome - 10 years follow up. J Korean Assoc Oral Maxillofac Surg. 1990; 16:106–113.
20. Min BI, Chun JH, Park YU. The nevoid basal cell carcinoma syndrome in a Korean family. J Korean Assoc Oral Maxillofac Surg. 1988; 14:37–43.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download