Abstract
Acrokeratosis paraneoplastica, or Bazex syndrome, is one of the paraneoplastic syndromes. The characteristic skin lesions include palmoplantar keratoderma, psoriasiform skin lesions, hyperpigmentation, and nail dystrophy. The most common associated neoplasms are squamous cell carcinoma of the upper respiratory tract and other kinds of tumors with cervical lymph node metastasis. A 63-year-old woman presented with an 11 month history of hyperkeratotic lesions on the palms and soles. Ten months before she had been diagnosed with adenocarcinoma of the colon and undergone a left hemicolectomy. We report a case of acrokeratosis paraneoplastica associated with colon cancer which persisted after removal of the primary cancer, but resolved with topical tretinoin treatment.
Acrokeratosis paraneoplastica (Bazex syndrome) is a rare disorder characterized by the presence of hyperkeratotic lesions involving the nose, ears, palms, and soles that appears in association with various malignancies. It was first reported by Gougerot and Grupper in 19221. Cutaneous lesions manifest as violaceous to erythematous psoriasiform lesions favoring the acral areas and including the hands, feet, nose, ears, and nails, with subungal hyperkeratotic and onycholytic lesions. Most of the affected patients are white males, approximately 40 years of age. The most common associated neoplasms are malignancies of the upper aerodigestive system, and other kinds of tumors with cervical lymph node metastasis2, but there are some cases that develop from other tumors in the prostate, bladder, thymus3, leg4, and breast5. A case of Bazexsyndrome associated with adenocarcinoma of the colon was first reported in 20006. No other cases of Bazex syndrome associated with colon cancer have been published in the English literature to date.
We report a case of acrokeratosis paraneoplastica associated with colon cancer which persisted after removal of the primary tumor, but improved with topical tretinoin treatment.
A 63-year-old Korean woman visited our dermatologic clinic with an 11 month history of hyperkeratotic lesions on the palms and soles. The routine laboratory tests, including a complete blood count, liver function tests, and urine analysis revealed values all within the normal range or negative findings. The physical examination revealed yellowish punctuate hyperkeratotic lesions on the palms and soles (Fig. 1A, B), as well as onycholysis of the toenails and fingernails (Fig. 1C, D). She had no subjective symptoms, such as pruritus or pain.
Ten months previously she was admitted to the hemato-oncologic department complaining of dyspnea and one episode of hematochezia. A mass was found on the descending colon by colonoscopy. Histopathologic examination of the mass revealed an adenocarcinoma with a moderate degree of differentiation, and she underwent a left hemicolectomy. The colon cancer was classified as stage T3N1M0, thus no adjuvant therapy was required. There was no family history of cutaneous disease or cancer.
The skin biopsy specimen from her right palm showed a thickened granular layer and scattered parakeratotic columns in the epidermis (Fig. 2A). There was acanthosis, and eosinophilic and vacuolar degeneration with exocytosis in the spinous layer (Fig. 2B). The histologic findings were compatible with keratosis palmaris et plantaris. Since the skin lesion preceded the development of colon cancer by 1 month, the patient was diagnosed with acrokeratosis paraneoplastica (Bazex syndrome).
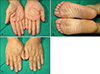
The skin lesions were treated with topical 0.025% tretinoin cream and the skin lesions resolved completely, except for the nail dystrophies (Fig. 3). There was no recurrence during 24 months of follow-up.
Acrokeratosis paraneoplastica is an example of a paraneoplastic syndrome which reflects an underlying malignancy. The clinical manifestations include palmoplantar keratoderma, violaceous psoriasiform papulosquamous lesions, hyperpigmentation, and nail dystrophy. Acrokeratosis paraneoplastica has a characteristic symmetric bilateral acral distribution, primarily affecting white males of French extraction over 40 years of age6. The most common associated neoplasms are squamous cell carcinoma of the upper respiratory tract and other tumors with cervical lymph node metastases. Also, various kinds of neoplasms in association with this syndrome are reported, including prostate adenocarcinoma3, bladder carcinoma, thymic carcinoma3, squamous cell carcinoma of the leg4, breast carcinoma5, hepatocellular carcinoma7, vulvar carcinoma3, lymphoma8, and multiple myeloma3. Thus far, only one case associated with colon cancer has been reported in the English literature6. Involvement of the nails appears in more than three-quarters of the cases, with subungal hyperkeratosis, ridging, thickening, onycholysis, pigmentation, anonychia, and onychomadesis9.
The histological findings are non-specific, most frequently showing hyperkeratosis, parakeratosis, and acanthosis. A perivascular lymphohistiocytic inflammatory infiltrate, vacuolar degeneration of the basal layer, and a few dyskeratotic keratinocytes may also be seen. Immunofluorescence studies of lesional skin from patients are usually negative, but Pecora et al10 described IgG, IgM, IgA, and C3 deposition along the basement membrane zone of lesional and non-lesional skin by direct immunofluorescence.
The psoriasiform skin lesions are known to precede the diagnosis of neoplasm in over 60% of the patients. Cutaneous manifestations follow the diagnosis of neoplasm in 15% of patients11. Bazex and Griffiths2 divided acrokeratosis paraneoplastica into three clinical stages. In stage 1, ill-defined erythema and scaling involves the fingers, nose, toes, nails, and the helices of ears. The tumor is asymptomatic during this stage. In stage 2, local symptoms occur. The skin lesions tend to spread to the palms, soles, and cheeks. If the tumor remains untreated, the skin lesions may expand to the arms, legs, and trunk in stage 310.
The pathogenesis of acrokeratosis paraneoplasica is still unknown. An immunologic mechanism based on the findings of immunofluerescence has been presented on the basis of immunofluorescence findings. Bazex and Griffiths2 suggested that cross-reactivity between tumor antigens and skin antigens leads to this syndrome. Some authors have demonstrated that the severity of clinical manifestations of acrokeratosis paraneoplastica parallel the serum concentrations of squamous cell carcinoma antigen, also suggesting an immunologic mechanism410. Bolognia7 reported that squamous cell lines produce TGF-α and insulin-like growth factor 1, which have a stimulatory effect on human keratinocytes. Politi et al12 disclosed that TGF-α levels in the urine of patients with acrokeratosis paraneoplastica decline markedly after surgery, together with clinical improvement.
Treatment with antibiotics, topical steroids, keratolytics, vitamin D, and PUVA achieve only transient remission2913. Despite some cases in which the cutaneous manifestations are completely healed by acitretin or etretinate without removal of the primary cancer1415, the treatment of skin lesions is directly related to eradication of the underlying neoplasm. Occasionally, a reappearance of the skin lesions signals the recurrence of the primary tumor or an appearance of skin lesions coincides with the development of metastatic disease9. Nail involvement usually persists long after the tumor has been successfully treated9.
In our case, the patient had persistent cutaneous manifestations after surgical extirpation of the neoplasm. We treated her with topical 0.025% tretinoin for about 2 months, which resulted in almost complete remission of the skin lesions, except for the dystrophic fingernails and toenails. We treated her with topical tretinoin because of the favorable response to many hyperkeratotic skin conditions, such as acanthosis nigricans or psoriasis. The effects of topical tretinoin on the epidermis include the induction of epidermal mitotic activity, the shedding of desmosomes and tonofibrils, and the production of a mucin-like material16. These actions affect cell growth, differentiation, and morphogenesis and alter cell cohesiveness17. Although are not studied yet, especially in acrokeratosis paraneoplastica, these actions can account for the improvement of various hyperkeratotic conditions. The case reported herein is a typical example of acrokeratosis paraneoplastica showing improvement with topical tretinoin.
Figures and Tables
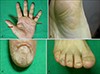 | Fig. 1Yellowish punctuate hyperkeratotic plaques on the left palm (A) and identical lesions on the right sole (B). Onycholysis on the finger nails (C) and toenails (D). |
References
1. Gougerot H, Grupper C. Dermatoses erythematosquameuse avec hyperkeratose palmo-plantaire, porectasies digitales et cancer de la langue latent. Paris Med. 1922; 43:234–237.
2. Bazex A, Griffiths A. Acrokeratosis paraneoplastica--a new cutaneous marker of malignancy. Br J Dermatol. 1980; 103:301–306.

3. Strobel ES, Bouveret C, Kohl PK. Acrokeratosis paraneoplastica of Bazex as an indicator for underlying squamous cell carcinoma of the lung. J Cancer Res Clin Oncol. 2006; 132:376–378.

4. Hara M, Hunayama M, Aiba S, Suetake T, Watanabe M, Tanaka M, et al. Acrokeratosis paraneoplastica (Bazex syndrome) associated with primary cutaneous squamous cell carcinoma of the lower leg, vitiligo and alopecia areata. Br J Dermatol. 1995; 133:121–124.

5. Akhyani M, Mansoori P, Taheri A, Asadi Kani Z. Acrokeratosis paraneoplastica (Bazex syndrome) associated with breast cancer. Clin Exp Dermatol. 2004; 29:429–430.

6. Hsu YS, Lien GS, Lai HH, Cheng YS, Hu CH, Hsieh MC, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000; 35:460–464.

8. Lucker GP, Steijlen PM. Acrokeratosis paraneoplastica (Bazex syndrome) occurring with acquired ichthyosis in Hodgkin's disease. Br J Dermatol. 1995; 133:322–325.

9. Valdivielso M, Longo I, Suarez R, Huerta M, Lazaro P. Acrokeratosis paraneoplastica: Bazex syndrome. J Eur Acad Dermatol Venereol. 2005; 19:340–344.

10. Pecora AL, Landsman L, Imgrund SP, Lambert WC. Acrokeratosis paraneoplastica (Bazex’ syndrome). Report of a case and review of the literature. Arch Dermatol. 1983; 119:820–826.

11. Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica). An analytic review. Medicine (Baltimore). 1991; 70:269–280.

12. Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol. 1993; 73:161–170.
13. Gill D, Fergin P, Kelly J. Bullous lesions in Bazex syndrome and successful treatment with oral psoralen phototherapy. Australas J Dermatol. 2001; 42:278–280.

14. Esteve E, Serpier H, Cambie MP, Armingaud P, Huet C, Penouil MH, et al. Bazex paraneoplastic acrokeratosis. Treatment with acitretin. Ann Dermatol Venereol. 1995; 122:26–29.
15. Wishart JM. Bazex paraneoplastic acrokeratosis: a case report and response to Tigason. Br J Dermatol. 1986; 115:595–599.

16. Darmstadt GL, Yokel BK, Horn TD. Treatment of acanthosis nigricans with tretinoin. Arch Dermatol. 1991; 127:1139–1140.

17. Blobstein SH. Topical therapy with tretinoin and ammonium lactate for acanthosis nigricans associated with obesity. Cutis. 2003; 71:33–34.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download