Abstract
Disseminated superficial porokeratosis (DSP) is a rare variant of porokeratosis, which is characterized histologically by cornoid lamella and clinically by central atrophy with elevated borders. DSP is usually associated with immunosuppressive states and hematopoietic malignancies, but rarely with malignancies of visceral organs. A 65-year-old male presented with numerous brownish macules with elevated borders on the trunk and limbs that had been present for 1 year. Before the visit to our clinic, gastric cancer was diagnosed at about the same time the skin lesions suddenly increased in size and number. Clinical and histopathological examination revealed that the lesions were consistent with DSP. We herein report a rare case of disseminated superficial porokeratosis that occurred in association with gastric cancer.
Porokeratosis is a group of disorders of keratinization that is characterized histologically by a parakeratotic column in the center of a keratin-filled invagination, called a cornoid lamella. Clinically, the basic lesion is sharply demarcated and hyperkeratotic with central atrophy. The pathogenetic mechanism of porokeratosis is still unknown, but porokeratosis is known as genetically heterogeneous disorder associated with UV light, trauma, infection, and immunosuppression1. Besides the classical porokeratosis of Mibelli, there are also five clinical variants: porokeratosis palmaris et plantaris disseminata (PPPD), linear porokeratosis, punctate porokeratosis, disseminated superficial porokeratosis (DSP), and disseminated superficial actinic porokeratosis (DSAP), all of which may be inherited by autosomal dominant mode.
DSP is a rare variant and is characterized by asymptomatic, small, annular papules that involve the trunk, genitalia, palms, and soles. Lesions range from 2 to 5 mm in size and an excess of 100 lesions may be present, but do not spare sun-protected area as in DSAP. The histological typical features of porokeratosis, cornoid lamella and vacuolated keratinocytes, are less pronounced in DSP than in classical porokeratosis of Mibelli. In many cases of DSP, the presence of immunosuppression has been an eliciting factor.
We report an unusual case of disseminated superficial porokeratosis in a patient with gastric cancer.
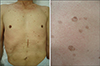
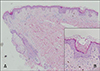
In July 2005, a 65-year-old man visited our department for evaluation of numerous round brownish macules and patches with slightly elevated rims on the trunk, face, and limbs that had developed about 1 year before his examination (Fig. 1). There was no associated pruritus or pain. A clinical diagnosis of disseminated superficial poro-keratosis (DSP), but not disseminated superficial actinic porokeratosis (DSAP), was made because the lesions were located not only in sun-exposed areas but also in sun-protected areas including the trunk. A skin biopsy specimen taken from the elevated rim of a lesion on the patient's abdomen revealed cornoid lamellae and some necrotic keratinocytes, which were consistent with porokeratosis (Fig. 2). Laboratory tests including liver function tests and kidney function tests showed no remarkable findings.
In June 2004, he had presented to the Department of Internal Medicine with abdominal discomfort. A diagnostic gastrofiberscope showed an elevated lesion on the stomach wall, and additional diagnostic evaluations including endoscopic biopsy and computed tomography revealed his lesion as early gastric cancer, poorly differentiated type. Subsequently he underwent surgical treatment, a subtotal gastrectomy, and no further treatment was needed.
At about the same time that early gastric cancer was diagnosed, disseminated skin lesions developed. He said he had had similar skin lesions for some time prior to his visit to our department. However, the previous lesions were scattered just on the trunk, not disseminated, and the rims of the lesions were not accentuated. Thus we determined that the DSP developed almost concurrently with diagnosis of gastric cancer.
He revisited our department in June 2007 with slightly improved lesions. During the 2 years following the gastrectomy, he had received no further treatment for the gastric cancer, and the skin lesions did not increase in size or number. They responded modestly to topical steroids.
hereditary factors and additional exogenous factors. An autosomal dominant mode of inheritance with reduced penetrance has been established for porokeratosis of Mibelli, PPPD, and DSP. In genetically predisposed skin, additional factors are assumed to trigger the clinical manifestations. Irradiation with ultraviolet light, organ transplantation23, bone marrow transplantation4, chronic hepatic failure5, renal failure6 and HIV infection7 are thought to be triggering factors.
In our patient, there was no family history of porokeratosis and no remarkable medical history except early gastric cancer. Furthermore, the skin lesions suddenly spread all over his body simultaneously with the diagnosis of gastric cancer. Therefore the DSP lesions seem to have occurred as a paraneoplastic phenomenon associated with gastric adenocarcinoma.
Several cases with rapid extension of DSP have been reported in patients with chemotherapy and bone marrow transplantation, which are associated with immunosuppression1. Moreover, DSP are often recognized after hematopoietic malignancies but there are few cases of development of DSP in patients with cancer of visceral organs. Lee et al8 reported DSP in a patient with cholangiocarcinoma and Takata et al9 reported hereditary non-polyposis colorectal cancer associated with DSP.
Gastric cancer is very common in Korea, being the leading cancer type accounting for 20.8% of all malignant neoplasms and the second leading cause of cancer deaths10. Several risk factors are involved in the development of gastric cancer including diet, gastritis, intestinal metaplasia, and Helicobacter pylori infection. In addition, inactivation of the p53 gene may play an important role in gastric tumorigenesis by loss of suppression of cancer cells11. A negative regulator of p53 function, mdm2, is also associated with molecular pathogenesis of gastric cancer12. In porokeratosis, too, overexpression of p53, decreased mdm2, and dysregulated cell cycle are pathogenically implicated13.
Shumack et al14 reported that hyperproliferative state of keratinocytes in porokeratosis is influenced by inflammatory mononuclear infiltrate, composed of helper T cells and suppressor T cells, located beneath the epidermis. Soluble factors released by activated T cells may provide a mitotic stimulus for overlying keratinocytes. Gastric cancer cells have been reported to secrete several cytokines including transforming growth factor (TGF)-α1516, interleukin (IL)-6 17, and IL-8 18. Thus, we speculate that these cytokines secreted by gastric cancer cells may play a role in the proliferation of keratinocytes, leading to increase in size and number of DSP lesions.
This case demonstrates the importance of thorough systemic work-up in patients with sudden appearance of DSP.
Figures and Tables
References
1. Kanitakis J, Euvrard S, Faure M, Claudy A. Porokeratosis and immunosuppression. Eur J Dermatol. 1998; 8:459–465.
2. Knoell KA, Patterson JW, Wilson BB. Sudden onset of disseminated porokeratosis of Mibelli in a renal transplant patient. J Am Acad Dermatol. 1999; 41:830–832.

3. Kanitakis J, Euvrard S, Claudy A. Porokeratosis in organ transplant recipients. J Am Acad Dermatol. 2001; 44:144–146.

4. Rio B, Magana C, Le Tourneau A, Bachmeyer C, Levy V, Hamont N, et al. Disseminated superficial porokeratosis after autologous bone marrow transplantation. Bone Marrow Transplant. 1997; 19:77–79.

5. Hunt SJ, Sharra WG, Abell E. Linear and punctate porokeratosis associated with end-stage liver disease. J Am Acad Dermatol. 1991; 25:937–939.

6. Hernandez MH, Lai CH, Mallory SB. Disseminated porokeratosis associated with chronic renal failure: a new type of disseminated porokeratosis? Arch Dermatol. 2000; 136:1568–1569.

7. Rodriguez EA, Jakubowicz S, Chinchilla DA, Carril A, Viglioglia PA. Porokeratosis of Mibelli and HIV-infection. Int J Dermatol. 1996; 35:402–404.

8. Lee HW, Oh SH, Choi JC, Chang SE, Lee MW, Choi JH, et al. Disseminated superficial porokeratosis in a patient with cholangiocarcinoma. J Am Acad Dermatol. 2006; 54(2 Suppl):S56–S58.

9. Takata M, Shirasaki F, Nakatani T, Takehara K. Hereditary non-polyposis colorectal cancer associated with disseminated superficial porokeratosis. Microsatellite instability in skin tumours. Br J Dermatol. 2000; 143:851–855.

11. Takashi M, Choitsu S, Toshiya S, Kohei M, Tohru U, Osamu N, et al. p53 gene mutations in human gastric cancer: wild-type p53 but not mutant p53 suppresses growth of human gastric cancer cells. Cancer Res. 1992; 52:4335–4341.
12. Gunther T, Schneider-Stock R, Hackel C, Kasper HU, Pross M, Hackelsberger A, et al. Mdm2 gene amplification in gastric cancer correlation with expression of Mdm2 protein and p53 alterations. Mod Pathol. 2000; 13:621–626.

13. Nelson C, Cowper S, Morgan M. p53, mdm-2, and p21 waf-1 in the porokeratoses. Am J Dermatopathol. 1999; 21:420–425.

14. Shumack S, Commens C, Kossard S. Disseminated superficial actinic porokeratosis. A histological review of 61 cases with particular reference to lymphocytic inflammation. Am J Dermatopathol. 1991; 13:26–31.
15. Tahara E. Growth factors and oncogenes in human gastrointestinal carcinomas. J Cancer Res Clin Oncol. 1990; 116:121–131.

16. Yonemura Y, Takamura H, Ninomiya I, Fushida S, Tsugawa K, Kaji M, et al. Interrelationship between transforming growth factor-alpha and epidermal growth factor receptor in advanced gastric cancer. Oncology. 1992; 49:157–161.

17. Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham DY. Relation between cytokines and Helicobacter pylori in gastric cancer. Helicobacter. 2001; 6:116–124.

18. Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992; 267:22506–22511.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download