Abstract
Melanoacanthoma is a rare benign mixed tumor of both keratinocytes and melanocytes. Although some authors said that it is a rare variant of seborrheic keratosis, it has clinical and histological features distinct from seborrheic keratosis. It has large dendritic melanin-laden melanocytes throughout all levels of epidermis showing a disruption of melanin transfer from the melanocytes to neighboring keratinocytes. However, it is difficult to distinguish melanocytes clearly from cutaneous pigment in immunohistochemical stain with usually used brown chromogen. We used chromogen with brick-red indicator product (VECTOR® NovaRED™) in S-100 and melan-A immunohistochemical staining to distinguish melanocytes from melanin laden keratinocytes. We suggest that the immunohistochemical staining using this novel chromogen may be useful in the diagnosis of melanoacanthoma.
Melanoacanthoma is a rare benign mixed neoplasm composed of melanocytes scattered throughout keratinocytic lobules, rather than being limited to the basal layer of these lobules. The term melanoacanthoma was first introduced by Mishima and Pinkus1 in 1960 to designate a benign skin tumor that consisted of proliferating melanocytes and keratinocytes. It has unique histologic features of large dendritic melanin-laden melanocytes throughout all levels of epidermis and a disruption of melanin transfer from the melanocytes to neighboring keratinocytes. However, it is difficult to distinguish melanocytes from melanin-laden keratinocytes when using brown chromogen which is usually used in immunohistochemical staining. We report a case of melanoacanthoma with immunohistochemical staining using a novel chromogen with brick-red indicator product, VECTOR® NovaRED™.
A 56-year-old woman was referred to our department with a skin lesion located on the abdomen since childhood. Physical examination revealed a 2 × 2 cm, well-demarcated, black-pigmented, irregular-shaped plaque on the abdomen (Fig. 1). She had no remarkable past or family history.
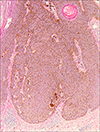
A punch biopsy was performed. Histopathology revealed hyperkeratosis, acanthosis, papillomatosis and pseudo-horn cysts in epidermis. The dermis showed mild lymphocytic infiltration and melanophages (Fig. 2). Immunohistochemical stains for melanocyte were performed using monoclonal antibodies for S-100 protein and melan-A as primary antibodies. Antigen-antibody complexes were visualized using a peroxidase-conjugated streptavidin with VECTOR® NovaRED™ as a chromogen. The tumor itself consisted largely of a proliferation of basaloid and squamous keratinocytes with low pigment content. Numerous large dendritic melanin-laden melanocytes were spread throughout the tumor at all levels of the stratum malpighii (Fig. 3). Electron microscopic findings showed a large number of dendritic melanocytes in the lesion with surrounding keratinocytes which were relatively free from transferred melanosomes.
We performed total excision and the patient has been under close observation for the past year without any evidence of recurrence.
Melanoacanthoma is uncommon benign cutaneous neoplasms that show combined proliferation of epidermal keratinocytes and large dendritic melanocytes. This tumor is found mainly in elderly people, more often in Caucasian patient than in other races, and both sexes are equally affected23. It is usually detected as a solitary, pigmented, verrucous-surfaced, round or oval plaque with a diameter ranging from a few millimeters to 10 cm, and is mainly found on the head, particularly the lips, and the trunk23. The differential diagnosis of melanoacanthoma includes seborrheic keratosis, pigmented basal cell carcinoma, malignant melanoma, and pigmented nevus4.
Histopathologic examinations show hyperkeratosis, papillomatosis, acanthosis and pseudohorn cysts. Keratinocytes consist of basaloid cells and squamous cells. Dopa or silver stains show melanocytes scattered throughout the entire epidermis and hyperinfiltrations of melanin. Electron microscopic studies reveal large highly dendritic melanocytes with abundant melanin granules in epidermis5. Some authors suggest that melanoacanthoma does not deserve a special denomination because the presence of a striking number of dendritic melanocytes at suprabasal levels is a frequent finding in every type of seborrheic keratosis, other than of the reticulated type6. However like most authors, we consider melanoacanthoma to be a clinically and histologically distinct entity1235. Recently, Kihiczak et al.5 suggested dendritic melanocytes are large and are situated not only in the suprabasalar area, but throughout all levels of epidermis in the true melanoacanthoma. In addition, unlike pigmented seborrheic keratoses, electron microscopic studies show a partial or complete disruption of melanin transfer from the highly dendritic melanocytes to neighboring keratinocytes, thus there are a few melanin in the cytoplasms of keratinocytes57.
We performed S-100 and melan-A immunohistochemical staining using VECTOR® NovaRED™ as a chromogen to distinguish melanocytes clearly from the melanin-laden keratinocytes, because the brown chromogen which is usually used can not provide sufficient contrast for the differentiation of positive immunostaining from the brown cutaneous pigment. This novel chromogen stains melanocytes red, which then can be differentiated from surrounding brown-colored melanin-laden keratinocytes. It has also been used in the cases to detect the micrometastases of melanoma or histopathological classification of minocycline-induced cutaneous hyperpigmentation89.
Two cases of melanoacanthomas have been reported in Korean literature, but to our knowledge there is no reported case of staining using this novel chromogen VECTOR® NovaRED™1011. We suggest that the immunohistochemical staining using VECTOR® NovaRED™ may be useful in the diagnosis of melanoacanthoma by facilitating the differentiation of melanocytes from surrounding melanin-laden keratinocytes.
Figures and Tables
References
1. Mishima Y, Pinkus H. Benign mixed tumor of melanocytes and malpighian cells. Melanoacanthoma: its relationship to bloch's benign non-nevoid melanoepithelioma. Arch Dermatol. 1960; 81:539–550.

2. Prince C, Mehregan AH, Hashimoto K. Large melanoacanthomas: a report of five cases. J Cutan Pathol. 1984; 11:309–317.

3. Vion B, Merot Y. Melanoacanthoma of the penis shaft. Report of a case. Dermatologica Basel. 1989; 179:87–89.
4. Sexton FM, Maize JC. Melanotic macules and melanoacanthomas of the lip. A comparative study with census of the basal melanocyte population. Am J Dermatopathol. 1987; 9:438–444.
5. Kihiczak GG, Centurion SA, Schwartz RA, Lambert WC. Giant cutaneous melanoacanthoma. Int J Dermatol. 2004; 43:936–937.

6. Simon P, Requena L, Sanchez Yus E. How rare is melanoacanthoma? Arch Dermatol. 1991; 127:583–584.

7. Schlappner OLA, Rowden G, Philips TM, Rahim Z. Melanoacanthoma: ultrastructural and immunological studies. J Cutan Pathol. 1978; 5:127–141.

8. Shidham VB, Qi DY, Acker S, Kampalath B, Chang CC, George V, et al. Evaluation of micrometastases in sentinel lymph nodes of cutaneous melanoma. Higher diagnostic accuracy with melan-A and MART-1 compared with S-100 protein and HMB-45. Am J Surg Pathol. 2001; 25:1039–1046.

9. Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol. 2003; 29:8–14.

10. Lee GS, Ahn KJ, Kim JM, Lee ES. A histopathologic study of the seborrheic keratosis. Korean J Dermatol. 1992; 30:76–80.
11. Kim JM, Kim JS, Cha MH, Lee CJ. A case of melanoacanthoma. Korean J Dermatol. 1984; 22:435–438.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download