Abstract
Background
A venous lake lesion is a venous ectasia that occurs on the exposed skin of elderly people. Although a number of therapies such as surgical excision, laser therapy, infrared coagulation, cryotherapy and sclerotherapy have been used to treat venous lakes, there is no guideline for treating this lesion.
Objective
The purpose of this study was to determine whether 0.5% sodium tetradecyl sulfate (STS) is effective for the treatment of venous lake lesions.
Methods
Twelve patients with venous lake lesions were enrolled In this study. After proper antiseptic preparation, 0.5% STS was slowly injected into each subject's lesion, and this was followed by immediate compression for 10 minutes.
Results
After treatment, all of the patients' lesions cleared completely. The average number of treatments was 2.15±1.28. Two patients experienced mild side effects such as light pain and paresthesia, and these soon disappeared. There were no serious side effects reported during treatment. The mean follow up period was 29.58±13.48 months.
A venous lake is a small, dark blue, slightly raised, soft lesion that occurs on the exposed skin of elderly people. This lesion is easily compressed, and it is mainly located on the face, ear, lip, neck, forearm and back of hand12. It is lined by a single layer of flattened endothelial cells and a thin wall of fibrous tissue3. Although it is usually asymptomatic, treatment may be required if there bleeding after trauma to this type of lesion, which can occur frequently, or if the lesion is cosmetically disfiguring. Numerous treatment modalities have been reported in the English Medical literatures, such as surgical excision4, laser therapy156, infrared coagulation7, cryotherapy8 and sclerotherapy910.
Sodium tetradecyl sulfate (STS) is a kind of sclerosing agent that's been used for the treatment of hemangioma and other vascular conditions111213. Intravenous injection of STS causes intimal inflammation and thrombus formation, and these conditions usually occlude the injected vein14. Thus, we designed this study to determine the efficacy of injectiong 0.5% STS for treating venous lake lesions.
We performed a clinical study on 12 patients who had venous lakes and who visited the Department of Dermatology, Hallym University Sacred Heart Hospital between June 2003 and December 2006. The male/female ratio was 5 males 7 females and their mean age was 52.91±13.38 years (range 32 to 76). Twelve lesions were on the lower lip and one lesion was on the upper lip and these lesions ranged in size from 2 to 10 mm. No patient had undergone previous treatment for their venous lake lesions.
Prior to treatment, informed consent was given and the venous lake was photographed. Age, gender, the underlying disease, the site, size, number and duration of the venous lake lesions and the previous treatments were recorded. To ascertain the exact diagnosis, we performed a 2 mm-punch biopsy on the lesion (Fig. 1). After proper antiseptic preparation, 0.5% STS was slowly injected to each patient's lesion by using an insulin syringe to flush the lesion clear of blood, and this was followed by immediate compression for 10 minutes. The amount of STS infusion depended on the size of each lesion, and the average STS infusion was 0.09±0.05 cc. The patients were given home dressings and they were instructed on daily care because a crust sometimes occurred.
Photographs of each patient were taken at the first visit, every injection day, and every follow up day to record the treatment progress. The patients were reexamined two weeks after the initial injection and then their treatment was repeatedly every two weeks until the lesion had completely cleared. Complete clearance was defined when there was no detectable color difference between the lesional area and the surrounding area and there was no recurrence of the lesion.
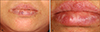
No patients were lost to follow-up. Five venous lakes cleared after a single treatment, four after two treatments, two after three treatments, one after four treatments and one after five treatments. The average number of treatments needed to achieve a complete cure was 2.15±1.28 (range 1 to 5) and the average infused volume of STS was 0.09±0.05 cc (range: 0.05 to 0.2). After treatment, all of the patients' lesions were completely cleared (Fig. 2). No patient experienced any recurrence with a mean follow up of 29.58±13.48 months (range 10~49 months) and there were no side effects such as swelling, inflammation, skin necrosis or hyperpigmentation. During injection, some patients felt light pain and paresthesia, but this soon disappeared. The details of the long term follow-up results are summarized in Table 1.
A venous lake lesion was first described by Bean and Walsh2 in 1956. It was previously called blueberry on the lip and senile hemangioma of the lip23. The lesion is a small, bluish-purple, slightly-raised, soft papule or nodule caused by a localized vascular dilatation. The size of a venous lake varies, but most of them measure 1 to 15 mm. The lesion is usually round or oval, but the larger ones may be irregular. It often occurs on sun-damaged skin and the most common sites are the face, ears and lips12.
Histopathologically, a few or multiple large, dilated and irregular thin-walled venules are located in the upper and middle dermisin the upper and middle dermis24. The venules are lined by a single layer of flattened endothelial cells and a thin wall of fibrous tissue3. In some instances, in place of the fibrous tissue, there is a thin irregular noncontinuous layer of smooth muscle15. This lesion is thought to occur as a result of deterioration in the connective tissue in the vascular adventitia, as well as in the dermis3.
Venous lakes have been previously treated by surgical excision4, laser therapy156, infrared coagulation7, cryotherapy8 and sclerotherapy910 has been previously reported. All of these methods were regarded as effective by the authors145678910. Although a patient with a venous lake is usually free of symptoms, it must sometimes be treated because of traumatic bleeding, as well as due to cosmetic concerns. Surgical excision is available for venous lakes, but this is considered to be more invasive than sclerotherapy and surgical excision harbors the risk of hemorrhage and scar. Laser therapy may result in skin atrophy, hyperpigmentation, a slight depression of the skin, and scarring and it also incurs high costs to the patient. Cryotherapy may also result in scarring and hyperpigmentation and hypopigmentation. All the lesions we evaluated in this study were small, which was one of the important factors for the choice of treatment. In addtion, sclerotherapy does not present the risk of hemorrhage, it is a less invasive method and it maintains a low cost.
Sclerotherapy refers to the injection of a foreign substance into the lumen of a vessel to cause endothelial and mural change, thrombosis, vessel wall necrosis and subsequent fibrosis16. It is a relatively simple, safe, quick and effective treatment for hemangioma and other related conditions. Sclerotherapy is effective for low-flow lesions because the injected sclerosant is diluted less rapidly and it remain in contact with the vessel walls for longer time17. A venous lake can be regarded as a low flow vascular lesion as the color of it fades on diascopy and the color reappears slowly after a release of pressure.
The two most commonly used kind of sclerosants for sclerotherapy are osmotic agents and detergents. Hypertonic saline and Sclerodex® (Omega Laboratories Ltd, Montreal, Canada) are osmotic agents that damage cells by shifting the water balance. Polidocanol, sodium morrhuate and STS are detergents that disrupt a vein's cellular membrane18. STS is a synthetic long-chain fatty acid salt of an alkali metal, and STS has detergent properties19. It has been approved by the US Food and Drug Administration (FDA) since 1946 and STS has a long record of safety and effectiveness20. Intravenous injection of STS causes intimal inflammation and thrombus formation, which occludes the injected vein and the partial or complete vein obliteration may or may not be permanent. The endothelial damage depends on the concentration of STS and this occurs immediately after injection, and the result is rapid thrombus formation14. This leads to the vascular sclerosis.
Although it is cost effective and safe with few major complications, 0.5% STS injection does have a few risks21. The side effects to be considered while using 0.5% STS are allergic reactions, pain, burning, itching and swelling. To avoid these negative reactions, we have to consider a patient's age, the presence of underlying disease, the patient's condition and the type and location of his or her venous lake before performing the treatment. In the presence of local infection, or if the patient has uncontrolled diabetes mellitus, injection therapy must be withheld22. In addition, when a patient uses an anticoagulant such as heparin, it is hard to treat this type of patient because thrombosis does not form very well23. The 1 cc insulin syringe needle should be precisely placed to the venous lake, and then 0.5% STS is injected slowly after being sure that the tip of the needle is located within the venous lake by aspiration of the venous blood. Thereafter, the treatment should be followed by immediate compression for 10 minutes. If the patient complains of severe pain, then the physician should stop the treatment because of the possibility of extravasation of the sclerosant. If the effect of the first injection is not sufficient, then further injections of sclerosing agent should be carried out after 2 to 3 weeks or at longer intervals.
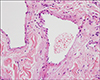
In this study, we performed a biopsy to make an exact diagnosis on the first visit (for all the patients except No. 3 and No. 10). In the upper dermis, close to the epidermis, they showed either one greatly dilated space or several interconnected dilated spaces filled with erythrocytes and these spaces were lined by a single layer of flattened endothelial cells and a thin wall of fibrous tissue(Fig. 1). There was no statistical difference in the number of treatments between the group with one greatly dilated space and the group with several interconnected dilated spaces (Wilcoxon two-sample test, p=0.7546).
Complete clearance was achieved for all the cases with no recurrence or complications. Our results with this treatment were better that those reported by the previous studies2425, although it is difficult to exactly compare between the studies because there are many factors that can affect the outcome, including the location, and size of the venous lakes and if skin biopsy was done926. Because there were no significant complications and all the lesions were cleared with an average of 2.15 treatments, we found that 0.5% STS injection for treating venous lakes was highly effective.
The limitation of this study was that the number of cases was small and there was no control group. We think a further study that will include a control group is needed in the future.
In conclusion, although many therapies exist for the eradication of venous lake lesions, the use of 0.5% STS injection appears to be a promising method that is safe, simple and effective.
Figures and Tables
Fig. 1
The dilated lumen is lined by a single layer of flattened endothelial cells and the lumen is filled with erythrocytes on the biopsy of case No. 4 (H&E, ×400).
References
1. Landthaler M, Haina D, Waidelich W, Braun-Falco O. Laser therapy of venous lakes (Bean-Walsh) and telangiectasias. Plast Reconstr Surg. 1984; 73:78–83.

3. Alcalay J, Sandbank M. The ultrastructure of cutaneous venous lakes. Int J Dermatol. 1987; 26:645–646.

4. Bu J, Shi H, Hu M, Liu H. Oral venous lakes: a clinicopathologic analysis of 20 cases. Zhonghua Kou Qiang Yi Xue Za Zhi. 2002; 37:33–35.
5. Gonzalez E, Gange RW, Momtaz KT. Treatment of telangiectases and other benign vascular lesions with the 577 nm pulsed dye laser. J Am Acad Dermatol. 1992; 27:220–226.

6. Neumann RA, Knobler RM. Venous lakes (Bean-Walsh) of the lips--treatment experience with the argon laser and 18 months follow-up. Clin Exp Dermatol. 1990; 15:115–118.

7. Colver GB, Hunter JA. Venous lakes: treatment by infrared coagulation. Br J Plast Surg. 1987; 40:451–453.

8. Suhonen R, Kuflik EG. Venous lakes treated by liquid nitrogen cryosurgery. Br J Dermatol. 1997; 137:1018–1019.

9. Kim WH, Kim SS, Kim CW, Kim KH, Kim KJ. Two cases of venous lake treated with a sclerosing agent. Korean J Dermatol. 2005; 43:71–73.
10. Kuo HW, Yang CH. Venous lake of the lip treated with a sclerosing agent: report of two cases. Dermatol Surg. 2003; 29:425–428.

11. Winter H, Drager E, Sterry W. Sclerotherapy for treatment of hemangiomas. Dermatol Surg. 2000; 26:105–108.

13. Seccia A, Salgarello M. Treatment of angiomas with sclerosing injection of hydroxypolyethoxydodecan. Angiology. 1991; 42:23–29.

14. Goldman MP, Kaplan RP, Oki LN, Cavender PA, Strick RA, Bennett RG. Sclerosing agents in the treatment of telangiectasia. Comparison of the clinical and histologic effects of intravascular polidocanol, sodium tetradecyl sulfate, and hypertonic saline in the dorsal rabbit ear vein model. Arch Dermatol. 1987; 123:1196–1201.

15. Elder DE, Elenitsas R, Johnson BL Jr, Murphy GF. Lever's histopathology of the skin. 9th ed. Philadelphia: Lippincott Williams & Wilkins;2005. p. 1025.
16. Goldman MP, Weiss RA, Brody HJ, Coleman WP 3rd, Fitzpatrick RE. Treatment of facial telangiectasia with sclerotherapy, laser surgery, and/or electrodesiccation: a review. J Dermatol Surg Oncol. 1993; 19:899–906.

17. Jackson IT, Carreno R, Potparic Z, Hussain K. Hemangiomas, vascular malformations, and lymphovenous malformations: classification and methods of treatment. Plast Reconstr Surg. 1993; 91:1216–1230.
18. Goldman MP, Bennett RG. Treatment of telangiectasia: a review. J Am Acad Dermatol. 1987; 17:167–182.

20. Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's dermatology in general medicine. 6th ed. New York: McGraw-Hill;2003. p. 2552–2553.
21. Frullini A, Cavezzi A. Sclerosing foam in the treatment of varicose veins and telangiectases: history and analysis of safety and complications. Dermatol Surg. 2002; 28:11–15.

22. Bong HW, Hann SK, Kim DI, Im SB. Clicically improved venous malformation by sclerotherapy. Korean J Dermatol. 1993; 31:992–998.
23. Goldman MP, Bergan JJ. Sclerotherapy: treatment of varicose and telangiectatic leg veins. 3rd ed. St. Louis: Mosby;2001. p. 151–240.
24. del Pozo J, Pena C, Garcia Silva J, Goday JJ, Fonseca E. Venous lakes: a report of 32 cases treated by carbon dioxide laser vaporization. Dermatol Surg. 2003; 29:308–310.

25. Bekhor PS. Long-pulsed Nd:YAG laser treatment of venous lakes: report of a series of 34 cases. Dermatol Surg. 2006; 32:1151–1154.

26. Mun JH, Yi JH, Park SH, Lee JS. Two cases of venous lakes. Korean J Dermatol. 2005; 43:849–851.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download