INTRODUCTION
Mast cells are specialized secretory cells distributed in connective tissues throughout the body. In the skin, mast cells are present in the papillary dermis, near the dermo-epidermal junction, in the sheath of epidermal appendages, and around the blood vessels and nerves of the subpapillary plexus1. Mast cells synthesize various kinds of mediators, some of which are preformed and stored in the granules, while others are synthesized and released without storage1. Among the mediators of mast cells, heparin, tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β), basic fibroblast growth factor (bFGF), tryptase, vascular permeability factor (VPF), and vascular endothelial growth factor (VEGF) have been suggested to contribute to various aspects of neovascularization2345. In addition, some reports have shown the increased number of mast cells and angiogenic mediators from mast cells are increased in hemangiomas and vascular malformations67. However, the simultaneous occurrence of mastocytoma and hemangioma is rarely reported. We describe a case of combined mastocytoma-hemangioma that occurred in a patient with urticaria pigmentosa.
CASE REPORT
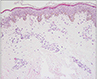
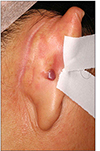
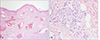
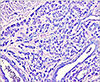
A 53-year-old woman was presented to our clinic to evaluate an asymptomatic skin lesion on the right ear that had developed two years before. Her past medical history was contributory to uticaria pigmentosa since childhood which had been treated by PUVA (Fig. 1). Bone marrow biopsy, which was performed before the skin lesion developed, revealed hypercellularity (90%) and hyperplasia (50%) of mast cells. Mast cells in bone marrow were positive for specific esterase, c-kit (CD117). Complete blood count and blood chemistry studies were normal ranged except for a slight thrombocytopenia. There was no family history for skin cancer or mastocytosis. On the physical examination, there was a 0.5×0.7 cm reddish to purplish soft papule on the posterior part of right ear (Fig. 2). But the Darier's sign was unsuspicious. Excisional biopsy was performed, and the specimen was fixed in 10% formalin and routinely embedded into paraffin for H&E and Giemsa staining. The H&E stained section revealed flattened rete ridge without basal hyperpigmentation in the epidermis. In the upper dermis, multiple dilated endothelial cell-lined vascular structures containing red blood cells were shown, and mast cells were densely infiltrated throughout the whole dermis including the intercapillary stroma (Fig. 3A). At the higher magnification view, the infiltrated mast cells were shown as round to cuboidal cytoplasm with central ovoid nuclei. They also had basophilic granules in the cytoplasm (Fig. 3B). In Giemsa staining, mast cells with abundant fine purple metachromatic cytoplasmic granules were observed (Fig. 4). The clinicopathologic features suggested a synchronous development of mastocytoma and hemangioma and we diagnosed this lesion as combined mastocytoma-hemangioma. During one year follow-up after excision, there was no evidence of recurrence.
DISCUSSION
Mastocytosis is characterized by the abnormal growth and accumulation of neoplastic mast cells in one or more organs. The skin is the most common site for the abnormal accumulation of mast cells, known as cutaneous mastocytosis8. Clinical categories of cutaneous mastocytosis, in order of frequency of occurrence, include urticaria pigmentosa, mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans (TMEP)8. It has been reported that mastocytoma had developed in a patient in association with another form of cutaneous mastocytosis. Okun and Bhawan9 reported a combined melanocytoma-mastocytoma in a patient of nodular mastocytosis, and Wood et al10. reported a case of fibrous mastocytoma in a patient with generalized cutaneous mastocytosis. Our patient had had urticaria pigmentosa since childhood and two years prior to examination, combined mastocytoma-hemangioma had developed as another histopathologic features from those previously reported.
The pathogenesis of developing combined mastocytoma-hemangioma is not clear, but there is some evidence that some mast cell mediators could induce neovascularization. Increased numbers of mast cells are often associated with rapidly growing capillaries in some conditions such as chronic inflammation or tumors2. An increased number of mast cells was found in the growing stages of strawberry hemangioma, and a significantly greater number of mast cells was found in angiolipomas than in classic lipomas611. These examples lead us to suggest that mast cells might be associated with the growth of new vessels. It has been reported that some mast cell mediators might contribute to various aspects of neovascularization. Taylor and Folkman12 showed that heparin could enhance angiogenesis induced by tumor extracts implanted in a chick embryo. It was revealed that heparin induced angiogenesis by significant migration of endothelial cells13. Heparin has also been found to have a very high binding affinity for endothelial cell growth factors, some of which are angiogenic14. Moreover, Qu et al.15 suggested that mast cells may be a significant source of tissue bFGF, one of the most potent angiogenic factors has been shown to induce angiogenesis, in angiogenic condition. Using specific antibodies, they demonstrated a marked spatial correlation between bFGF immunoreactivity and mast cells in cutaneous hemangioma. Such correlation indicates that mast cells could be a source of tissue bFGF during neovascularization. Boesiger et al.5 reported that human mast cells can produce and secrete VPFs and VEGFs, which are important regulators of neovascularization. It was revealed that mast cells can secrete VPF and VEGF upon activation via the Fcε receptor (FcεRI). Moreover, they found that the IgE-dependent secretion of VPF and VEGF can be significantly increased in mast cells, which undergo IgE-dependent upregulation of their surface expression of FcεRI. In particular, they found that neoplastic mast cell lines can secrete greater amounts of VPF and VEGF than nonneoplastic mast cells. According to these findings, we suggest that the development of hemangioma could be induced by mast cell mediators such as heparin, basic fibroblast growth factor, vascular permeability factor, and ascular endothelial growth factor, which have angiogenic properties.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download