Abstract
Background and Objectives
Recently, the swinging door and grafting techniques have been heavily used for straightening and holding the caudal septum. However, reconstructive septoplasties require more extensive dissection of septal structures. Extensive anatomical dissection and complicated procedures may affect the probability of postoperative bleeding and infection.
Materials and Method
We retrospectively reviewed the records of 141 consecutive patients who underwent septal surgeries from February 2013 to December 2015. The patients were classified into two groups according to surgical technique: those who underwent submucous resection with or without endoscopy were classified as the “resection” group, while those who underwent the swinging door or batten graft technique were classified as the “reconstruction” group. The resection and reconstruction groups were matched using the propensity score. The incidence of postoperative septal abscesses (PSAs) was analyzed between the two groups.
Results
For the two groups, 36 patients were matched with 36 patients (1:1) using the propensity score. Of the 72 patients, PSAs developed in 5 patients (6.9%). One patient was in the resection group (2.8%), while the other four patients were in the reconstruction group (11.1%). However, the incidence of PSAs was not significantly higher in the reconstruction group according to Fisher's exact test (p=0.164).
Septoplasty is one of the most common surgeries in otolaryngology. Submucous resection (SMR) is still practiced as a prevalent technique of resecting a deflected segment of the septum. With the introduction of endoscopy, more precise SMR is possible under superior vision.1) However, not all septal deviations can be corrected with this resection technique. A reconstruction technique must be used when correcting the framework of the cartilaginous septum, where is placed approximately a 1 cm dorsal and 1 cm caudal of the septum, termed “L-strut”. Resecting the L-strut area of the septum may sacrifice stability of the nose. Recently, swinging door or grafting techniques have been prevalently used to straighten and hold the caudal septum.2)3) But, these reconstructive septoplasties require more extensive dissection of septal structures. Bilateral mucosa elevation is a necessary procedure in reconstructive septoplasty, but it was avoided in the past for fear of reducing the survival of the cartilaginous septum.
Septal infections as a postoperative complication are not commonly reported, and they usually start from a preceding septal hematoma.4) Bleeding entrapped between the mucosal flap and septal cartilage can soon become infected and hinder blood supply to the cartilage, which causes nasal obstruction, swelling of the septum, pain, and fever. If a septal abscess is not properly treated, septal perforation, cavernous sinus thrombophlebitis, and saddle nose deformity can occur.4)5)
Extensive anatomical dissection and complicated procedures may affect the chance of postoperative bleeding chance and the infection rate. Therefore, this study aims to compare the incidence of septal infection between resection and reconstructive septoplasty techniques.
Retrospectively, 148 consecutive patients were recruited who underwent septal surgeries from February 2013 through December 2015 at the Eulji Medical Center, Daejeon, Korea, by a single surgeon (M. S. Choi). This study was reviewed and approved by the Institutional Review Board of the Eulji Medical Center (EMC 2015-10-002). In total, 141 patients of 148 who were followed up for more than 3 months were included in the study. They were classified into two groups by surgical techniques: an SMR with or without an endoscopy was classified as the “resection” group and a swinging door or batten graft was classified as the “reconstruction” group. Retrospectively, medical records and the incidence of postoperative septal abscesses (PSAs) were analyzed between the groups. In addition, clinical records of the patients who developed PSAs were analyzed.
In order to control the comfounding factors such as, Age, Sex, comorbidity (Diabetes, Hypertension), Allergy, Revision surgery, combined rhinoplasty, and operating time, logistic regression between two groups was performed and the Propensity scores were obtained. Two groups were matched one to one ratio with similar Propensity score. Each resection and reconstruction groups composed of 36 patients.
The statistical analysis was performed using SPSS 12.0 (SPSS, Inc., Chicago, IL, USA). The incidence of PSAs was analyzed between the two groups, using Fisher's exact test. The Propensity scoring match was validated by using a Paired t-test. A value of p<0.05 was accepted as statistically significant.
Prior to all septal surgeries, both nostrils were disinfected with betadine-soaked cotton, and both nasal cavities were irrigated with a diluted betadine solution (approximately 100–200 cc). Immediately after surgery and daily, 3 g of cephazedone (Kukje, Inc., Seongnam, Gyeonggi-do, Korea) was injected intravenously until packings were removed (nasal cavities were usually packed 2 days after surgery). There were no differences in medication for each septal surgery. A drain site was made to the unilateral mucoperichondrial flap by stab incision placed posterior to the L-strut area when there were no tears in both mucosal flaps during elevation. If severe mucosal damage occurred, a thin silastic sheet was applied to the damaged side and secured with vicryl 4-0 (Ethicon, Inc., Somerville, NJ, USA) without tension, then packed lightly with Vaseline-soaked gauze or Merocel sponges (Medtronic-Xomed, Inc., Jacksonville, FL, USA). When the septal surgeries were accompanied with open rhinoplasty, a marginal and a columellar incision was made and a skin envelop was elevated.
A hemitransfixion incision or a Killian incision was made to the one side (usually concave side) of nasal septum. After elevation of the unilateral mucoperichondrial flap, resection of the deflected segment of cartilage and bone was performed, preserving the L-strut area (Fig. 1). The Killian incision site was left open, and the hemitransfixion incision site was sutured with ethilon 5-0 (Ethicon, Inc., Somerville, NJ, USA).
Generally, a Killian incision was made to the concave septal mucosa under endoscopy, and a horizontal incision adjacent to the lesion was made occasionally when addressing only septal spurs. Elevation of unilateral mucosal flaps was performed being careful not to tear the mucosa. The resection procedure was the same as the SMR except with using an endoscope (Fig. 1). An incision site was left open.
A hemitransfixion incision was made to the concave side of the nasal septum. Through this incision, bilateral mucosal flaps were elevated. To swing superiorly, a cartilaginous septum was firstly separated from the bony septum, preserving at least 1 cm from the dorsum, then separated inferiorly from the maxillary crest and an anterior nasal spine (ANS) (Fig. 1). Precise excision of vertical excess was performed to allow the deviated septum to be realigned to the midline. Finally, the newly positioned septum was secured tightly to the periosteum of the ANS or to the ANS itself held by sharp punch with polydioxanone (PDS) 4-0 sutures (Ethicon). In some cases, cartilaginous septum not excised of excess portion was flipped over the ANS, which act as a doorstop. The incision site was sutured by ethilon 5-0. Finally, two point quilting sutures were applied to the septum by vicryl 4-0.
A hemitransfixion incision was made to the concave side of nasal septum. Through this incision, bilateral mucosal flaps were elevated. A rectangular shape of cartilage or bone was harvest, preserving the L-strut. A cartilaginous septum was completely separated from the maxillary crest and the ANS, and it was tightly secured midline, as in the swinging door technique. A bone graft from the perpendicular plate of ethmoid or cartilage from the septum was placed on the concave caudal septum to hold the septum straight, and it was sutured by PDS 4-0 or 5-0 (Fig. 1). The incision site was sutured by ethilon 5-0. Finally, two-point quilting sutures were applied to the septum by vicryl 4-0.
A total of 141 eligible patients were enrolled this study and followed up for more than 3 months. The resection group adopting an SMR with or without endoscopy included 83 males and 13 females, with a mean age of 37.9 years (range: 17 to 78 years). The reconstruction group adopting a swinging door or caudal batten graft included 43 males and 2 females, with a mean age of 36.5 years (range: 17 to 63 years). Of the 141 patients, the resection group and reconstruction were matched 36 patients to 36 (1:1) by the similar Propensity score. After matching with the Propensity score, two groups showed no significant differences of sex, age, hypertension, diabetes, allergy, revision surgery, combined rhinoplasty, and operative time (Table 1). The Propensity scores between newly matched groups were not significantly different by Paired t-test (p-value=0.235).
Of the 72 patients, PSAs were developed in 5 patients (6.9%). One patient was in the resection group (2.8%), and four patients were in the reconstruction group (11.1%) (Fig. 2). But, the incidence of PSAs was not significantly higher in the reconstruction group by Fisher's exact test (p=0.164).
Information regarding the eight patients who developed PSAs is summarized in Table 2. Bacteria isolations were identified in half of the eight patients with PSAs (50%). Methicillin-resistant Staphylococcus aureus (MRSA) was isolated in one patient (case 1). Serratia marcescens, Pseudomonas aeruginosa, and Staphylococcus aureus were isolated in the wound culture of three patients (Table 2). The duration of the time to diagnose the septal infections was longer in the reconstruction group (approximately 24.8 days) than in the resection group (approximately 6 days). In addition, the color of fluid entrapped in the septum was purulent when the infection was diagnosed later (>10 days) but was turbid when the infection was diagnosed earlier (<10 days) (Table 2). Granulation tissue on the mucosa of the septum was identified in four patients (50%) (Fig. 3). When incising around the granulation tissue, purulent discharge was drained in all four patients.
PSAs have been considered uncommon. Furthermore, there were few reports in the literature about the incidence of PSAs, and they reported different incidences of it. Yoder and Weimer reported only 5 postoperative infections (0.48%) in 1040 patients undergoing either septoplasty or septorhinoplasty using an endonasal approach.6) Makitie et al. reported 12 postoperative septal abscesses (12%) in 100 patients undergoing septoplasty under local anesthesia by resecting the septal deformities and by reconstructing the septum.7) Our study revealed eight PSAs (5.7%) in 141 septal surgeries. However, the incidence of septal infections was different according to the surgical techniques of the septoplasty. The incidence of PSAs was low (2.8%) in the resection group and high (11.1%) in the reconstruction group, which is similar to the findings by Makitie et al. However, there are some reports indicating very low rates of septal infections even when using reconstructive septoplasty, such as batten graft, cutting and suture technique, and extracorporeal septoplasty.8)9) To the best of our knowledge, there was no English literature comparing PSAs according to the surgical techniques of the septoplasty.
The authors performed logistic regression analyses and Propensity score matching to minimize comfounding factors such as, age, sex, operation time, combined surgeries, allergy, revision surgery, diabetes mellitus, and hypertension.
Although there was no significant difference, the frequency of PSAs in the reconstructive group was higher than resection. The reasons for this are supposed as follows. The first is an extensive anatomical dissection; it is essential for reconstructive septoplasty to elevate bilateral mucosal flaps and to disarticulate to the maxillary crest and ANS, which is not essential for SMR. The location of incisions is different between reconstruction septoplasty and resection. PSAs mainly occur due to a preceding postoperative hematoma. When disarticulating the septum from the maxillary crest, sometimes, significant bleeding due to injury of the greater palatine artery can occur, which requires electrocauterization. In addition, bleeding is common around the ANS, and an incision site close to the skin is an essential site to dissect extensively for reconstructive septoplasty.
The second is the amount of dead space left after surgery. The amount of dead space is also closely related with the degree of the anatomical dissection. A quilting suture to the septum does not guarantee complete coaptation of the mucosa to the septum. Dead spaces can exist between the newly realigned septum and maxillary crest. However, the dead space is only between the unilateral mucosal flap and cartilaginous septum in the SMR. In our study, the septal infections were mostly found on both sides across the septum in reconstructive septoplasty but were found unilaterally in the SMR (Table 3). Furthermore, a small amount of bleeding or pus pooled in the dead spaces after reconstructive septoplasty may be difficult to find in the early period. A caudal septum that is grafted with cartilage or bone normally appears mildly swollen, which can make it difficult to differentiate a normal postoperative shape of the caudal septum or one due to septal infection. However, in SMR cases, septal infections can be detected easily because of the distinct imbalance of the septal mucosal swelling compared with the normal side, when inspected with an anterior rhinoscope. In our study, it took approximately 25 days in reconstructive septoplasty and approximately 6 days in SMR to detect and diagnose septal infections.
The incidence of postoperative septal abscesses or hematoma can be reduced if the surgical site drains well. In our 8 cases of PSAs, all septal infections developed in the caudal septum. Commonly, an incidental mucosal tear can occur when elevating the mid-portion of a septal mucosa that has severe curvature or spur. Sometimes, an intentional stab incision can be made to the intact mid-portion mucosa for drainage. However, a mucosa of the caudal portion of septum is so thick as not to tear usually during elevation, and it is not a usual site to place an intentional stab incision. The authors recently placed a stab incision and inserted a thin silastic drain to the inferior portion of the caudal septum before the end of the surgery and removed the drain 5 to 7 days after surgery.
We performed additional surgery to batten the weakened L-strut with the auricular cartilage to case 3, 4, and 5 patients to strengthen the septal support after controlling infections with incision and drainage and infusion of susceptible antibiotics. The remaining five patients with infections were treated the same as above without additional surgery. All eight patients with infections were followed up over 12 months and were confirmed to have no nasal obstruction and no deformity of nose shape. Septal infections can progress to abscesses and cause necrosis of septal cartilage that can yield perforation of the septum and saddle nose deformity, without proper treatment. In those cases, supportive or reconstructive surgery using an auricular or rib cartilage is necessary after controlling infections.10)
In our study, MRSA was isolated in one patient (case 1). Abuzeid et al. also reported one case of MRSA after septorhinoplasty with culture of postoperative septal abscess, and they recommended applying intranasal mupirocin ointment 5 days before septorhinoplasty for high-risk groups (e.g., hospital care workers, immunocompromised people, and the elderly).11) S. aureus is normal flora of the skin and a commonly isolated species after septorhinoplasty. S. aureus can be isolated in the anterior nares of approximately 60% of the total population.11) Other researchers reported that S. aureus is the most common pathogen isolated in PSAs.7)12) In our study, S. aureus was isolated in only one patient (12.5%). There is a report that diabetes mellitus can increase colonization of S. aureus.13) Serratia marcescens (belonging to Enterobacteriaceae) was cultured in one patient in our study (Table 2). Serratia marcescens is considered a hospital-acquired infection, along with MRSA.
There was a report using blood culture that bacteremia can be occur transiently. It is 0% cultured before septorhinoplasty but 15–16.9% cultured from the end of the surgery until packings are removed.14) Another study reported that bacteremia was common in open septorhinoplasty (13.3%) than septolasty (3.3%) after surgery.15) It has been controversial to use of prophylactic antibiotics to reduce infection in nasal surgeries. Andrews et al., in a randomized study of 164 patients undergoing complex septorhinoplasty, reported that infection rates between the prophylactic (7%) and postoperative (11%) use of antibiotics groups were not significantly different.16) However, they recommended the use of prophylactic antibiotics for patients undergoing complex septorhinoplasty.16)
One patient (case 8) complained of facial pain and headache when followed 9 days after the SMR. The patient discontinued antiplatelet drugs for 5 days before surgery and took it again after surgery for prevention of cardiovascular accidents. When incising the original incision site of the septum, an old hematoma with mild foul odor was drained, and the symptoms of the patient disappeared. Recently, because of the increased average life span, antiplatelet drugs or anticoagulant agents have been widely prescribed to prevent or treat ischemic vascular diseases.17) Thus, attention should be paid to patients with a hemorrhagic tendency after septoplasty.
Granulation tissue found on the septum after septoplasty can be an important hallmark of septal infections (Fig. 3). Granulation tissue was found in four of our cases that were diagnosed later (>10 days after surgery) with an infection (Table 2). It bled easily and purulent discharge was drained in all four cases when incised around the granulation tissue.
Figures and Tables
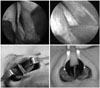 | Fig. 1Different techniques to correct deviated nasal septum. Classic submucous resection (top left). Endoscopic septoplasty (top right). Caudal batten graft (bottom left). Swinging door technique (bottom right). |
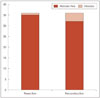 | Fig. 2Comparison of postoperative septal abscesses rates between resection and reconstructive groups matched by the Propensity score. |
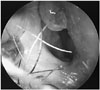 | Fig. 3Granulation tissue that bled easily was seen on the mucosa of the septum by the right side. Purulent discharge was drained when incised around the granulation tissue on the septum. |
Table 2
Description of patients who developed postoperative septal infections

N: number, D: duration (days), Gr: granulation tissue, o-rhino: open rhinoplasty, c-rhino: closed rhinoplasty, ESS: endoscopic sinus surgery, MRSA: Methicillin resist Staphylococcus aureus, S. marcescens: Serattia marcescens, P. aeruginosa: Pseudomonas aeruginosa, S. aureus: Staphylococcus aureus, HTN: hypertension, DM: diabetes mellitus, anti-PLT: antiplatelet drugs
References
2. Jin HR, Won TB. Septoplasty; Current concept and technique. J Rhinol. 2008; 15(1):13–29.
3. Kim YD. Septoplasty and turbinoplasty; Current concept and technique. J Rhinol. 2012; 19(1):19–28.
6. Yoder MG, Weimert TA. Antibiotics and topical surgical preparation solution in septal surgery. Otolaryngol Head Neck Surg. 1992; 106(3):243–244.

7. Mäkitie A, Aaltonen LM, Hytönen M, Malmberg H. Postoperative infection following nasal septoplasty. Acta Otolaryngol Suppl. 2000; 543:165–166.

8. Lee SB, Jang YJ. Treatment outcomes of extracorporeal septoplasty compared with in situ septal correction in rhinoplasty. JAMA Facial Plast Surg. 2014; 16(5):328–334.

9. Gubisch W. Extracorporeal septoplasty for the markedly deviated septum. Arch Facial Plast Surg. 2005; 7(4):218–226.

10. Pribitkin EA, Ezzat WH. Classification and treatment of the saddle nose deformity. Otolaryngol Clin North Am. 2009; 42(3):437–461.

11. Abuzeid WM, Brandt MG, Moyer JS, Baker SR. Methicillin-resistant Staphylococcus aureus-associated infections following septorhinoplasty. Facial Plast Surg. 2012; 28(3):354–357.

12. Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997; 10(3):505–520.

13. Yoo DB, Peng GL, Azizzadeh B, Nassif PS. Microbiology and antibiotics prophylaxis in rhinoplasty: a review of 363 consecutive cases. JAMA Facial Plast Surg. 2015; 17(1):23–27.

14. Kaygusuz I, Kizirgil A, Karlidağ T, Yalçin S, Keles E, Yakupogullari Y, et al. Bacteriemia in septoplasty and septorhinoplasty surgery. Rhinology. 2003; 41(2):76–79.
15. Okur E, Yildirim I, Aral M, et al. Bacteremia during open septorhinoplasty. Am J Rhinol. 2006; 20(1):36–39.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download