Abstract
Background and Objectives:
Olfactory disturbance is a major sinonasal symptom of chronic rhinosinusitis (CRS) with nasal polyps (NP). The aim of this study was to investigate the relationship between the severity of nasal polyposis and olfactory dysfunction in CRS. Subjects and Method: A total of 277 subjects with CRS were included in this study. All participants were divided into four groups according to the size and degree of the nasal polyposis: the control group (no polyp; n=79); NP (nasal polyp) group I (both or unilateral simple polyposis; n=85); NP group II (unilateral diffuse polyposis; n=66); and NP group III (bilateral diffuse polyposis; n=47). We analyzed the relationships between the severity of nasal polyposis and olfactory dysfunction using both the Korean Version of the “Sniffin’Sticks” test (KVSS Test) II and the Questionnaire of Olfactory Disorders (QOD).
Results:
The KVSS Test II TDI score was significantly decreased in the bilateral diffuse polyposis group (NP group III= 15.62±13.39) compared to the other polyp groups [control group=25.04±9.67 (p<0.001); NP group I=21.67±11.18 (p=0.005); NP group II=21.51±10.85 (p=0.008)]. However, there were no significant differences in the KVSS Test II TDI score between the control group and NP groups I and II. For the QOD_NS score, only NP group III (11.51±9.87) had significantly increased values compared to the control group [8.42±12.27 (p=0.015)].
REFERENCES
1). Alt JA, Smith TL. Chronic rhinosinusitis and sleep: a contemporary review. International Forum of Allergy & Rhinology. 2013; 3:941–9.

2). Thomas AJ, Orlandi RR, Ashby Se, Mace JC, Smith TL, Alt JA. Nasal obstruction has a limited impact on sleep quality and quality of Life in patients with chronic rhinosinusitis. Laryngoscope. 2016; 126:1971–6.

3). Thompson CF, Kern RC, Conley DB. Olfaction in endoscopic sinus and skull base surgery. Otolaryngologic Clinics of North America. 2015; 48:795–804.

4). Choi HG, Lee HJ, Shin HW, Lee JM, Lee CH, Mo JH. Correlation between olfactory threshold test, olfactory identification test and subjective symptoms. Korean J Otorhinolaryngol-Head Neck Surg. 2008; 51:1015–9.
5). Choi SH, Kim ST, Park HM, Moon KH, Jung JH, Cha HE. Analysis of chracteristics and steroid effects in olfactory dysfunction patients. J Rhinol. 2016; 23:39–43.
6). Lee SH. The Pathogenesis of Nasal Polyp. J Rhinol. 2005; 12:10–5.
7). Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nick-las RA, et al. Rhinosinusitis: Developing guidance for clinical trials. Otolaryngol Head Neck Surg. 2006; 118:17–61.

8). Kim JM, Jeong MS, Shin DH, Seol JH, Hong SC, Cho JH, et al. Olfactory identification test using familiar distracters for koreans. Clinical and Experimental Otorhinolaryngology. 2014; 7:19–23.

9). Frasnelli J, Hummel T. Olfactory dysfunction and daily life. Eur Arch Otorhinolaryngol. 2005; 262:231–5.

10). Hong SC, Ahn JY, Cho JH, Lim DJ, Park GH. Clinical analysis of the etiology of korean olfactory disorders. Korean J Otorhinolaryngol-Head Neck Surg. 2008; 51:717–21.
11). Rhyoo C, Jung MK, Lee JH. The clinical significance of Lund-Mack-ay CT staging system in assessing the severity of chronic rhinosinusitis. Korean J Otolaryngol. 2001; 44:837–41.
12). de Loos DAED, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2013; 123:57–63.
13). Olsson P, Stjarne P. Endoscopic Sinus surgery improves olfaction in nasal polyposis, a multi-center study. Rhinology. 2010; 48:150–5.

14). Simopoulos E, Katotomichelakis M, Gouveris H, Tripsianis G, Liva-ditis M, Danielides V. Olfaction-associated quality of life in chronic rhinosinusitis: adaptation and validation of an olfaction-specific questionnaire. Laryngoscope. 2012; 122:1450–4.

15). Hong SH, Yoo YS, Kim ES, Kim SC, Park SH, Kim JK, et al. De-velopment of KVSS test (Korean Version of Sniffin' Sticks Test). Korean J Otolaryngol. 1999; 42:855–60.
16). Altundag A, Salihoglu M, Tekeli H, Saglam M, Cayonu M, Hummel T. Lateralized differences in olfactory function and olfactory bulb volume relate to nasal septum deviation. Journal of Craniofacial Surgery. 2014; 25:359–62.

17). Garneau J, Ramirez M, Armato SG 3rd, Sensakovic WF, Ford MK, Poon CS, et al. Computer-assisted staging of chronic rhinosinusitis correlates with symptoms. Int Forum Allergy Rhinol. 2015; 5:637–42.

Fig. 1.
Meltzer score used to classify nasal polyp grade. 0: no visible NP, 1: small amount of polypoid disease confined within the middle meatus, 2: multiple polyps occupying the middle meatus, 3: polyps extending beyond the middle meatus, 4: poly.
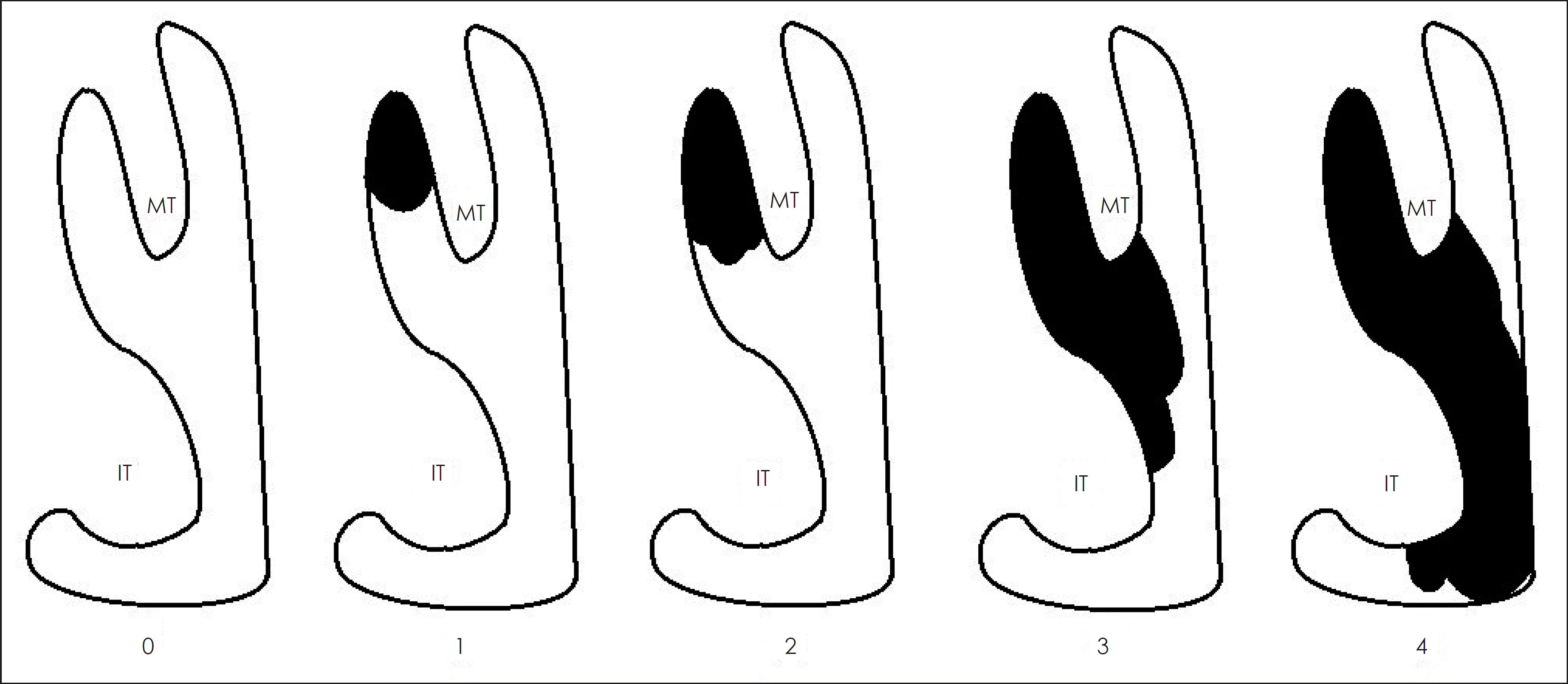
Fig. 2.
NP groups and KVSS TDI. Bilateral diffuse polyposis group ( NP group IV, KVSS Test II TDI score=15.62±13.39) have significantly decreased in KVSS Test II TDI score compared to other polyposis groups [ control group=25.04±9.67 ( p=0.008)]; NP gro.
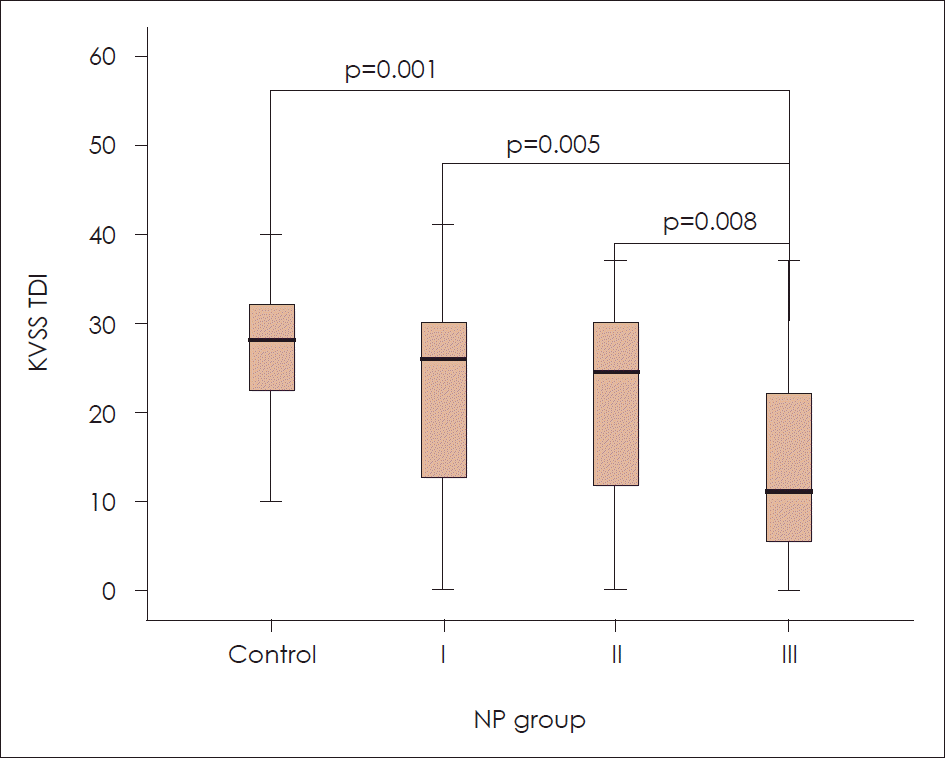
Table 1.
Demographic data, PNIF, Lund-Mackay score, KVSS II of NP groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download