Abstract
Background and Objectives
Obstructive sleep apnea syndrome(OSAS) is characterized by repeated apnea, hypopnea, and micro-arousals during sleep. Among various treatment modalities of OSAS, continuous positive airway pressure(CPAP) treatment is the most effective and successful. The aim of this study was to compare efficacy and safety of newly developed Korean CPAP with standard CPAP in OSAS patients.
Materials and Method
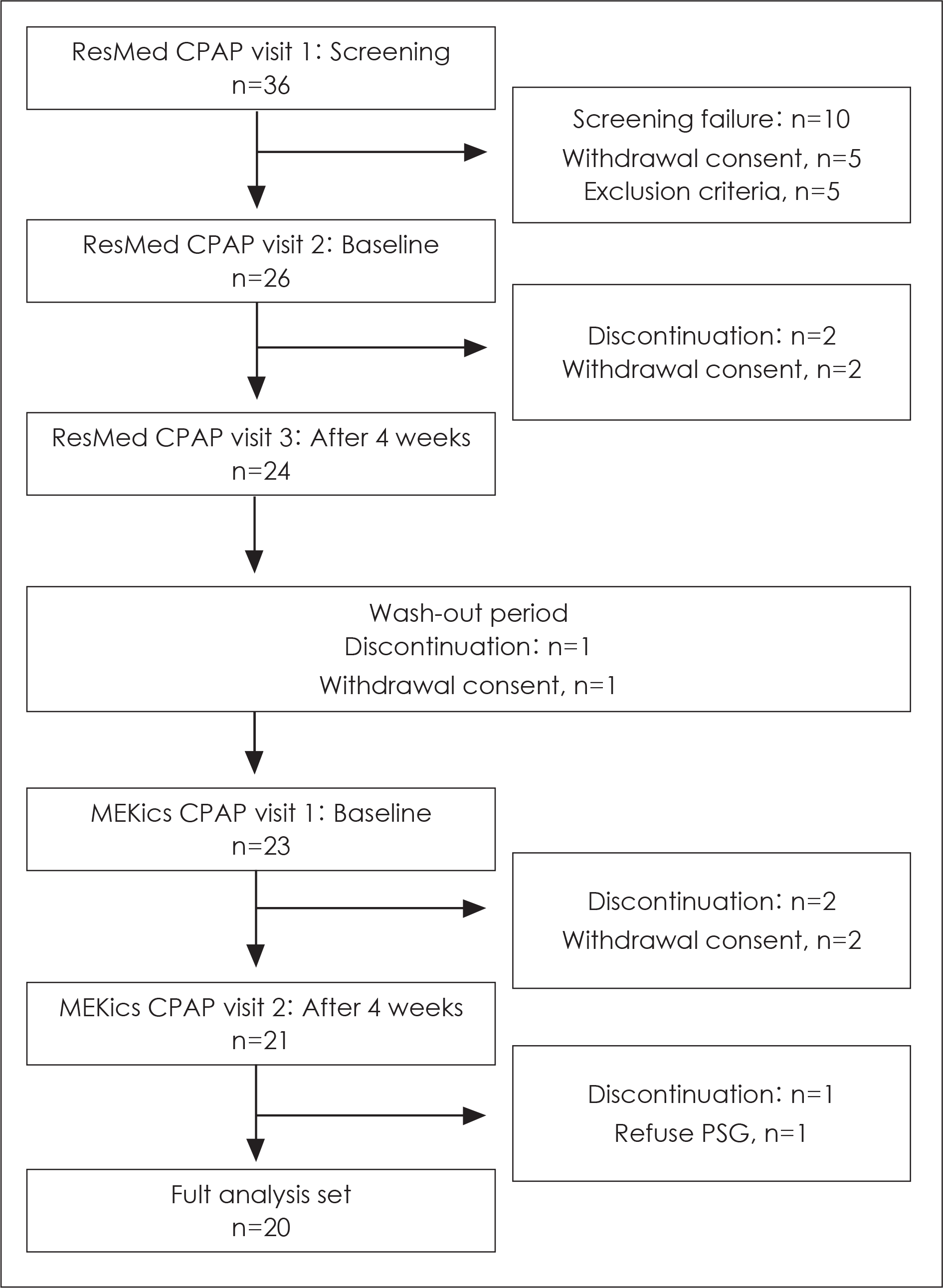
In total, 20 of 26 recruited OSAS patients completed the study. All subjects first used the standard CPAP for 4 weeks. After an at least 2 week washout period, the subjects used the newly developed CPAP for 4 weeks. Polysomnography, questionnaires associated with sleep, lipid profile, pulmonary function test, cardiac marker, and physical examinations were evaluated at baseline and were followed-up after each treatment.
Results
After treatment with the newly developed CPAP, the apnea-hypopnea index was decreased from 53.2/hr to 2.5/hr and was equivalent to that of the standard CPAP. Most of the changes in questionnaire scores, laboratory findings, and physical examinations after newly developed CPAP treatment were equivalent to those with standard CPAP. No serious adverse events were observed during the study.
Go to : 
References
1). Chung YS. Pathogenesis of Obstructive Sleep Apnea. J Rhinol. 2009; 16:87–90.
2). Mo JH. Obstructive Sleep Apnea and Systemic Diseases. J Rhinol. 2013; 20:8–12.
3). Kim J, In K, Kim J, You S, Kang K, Shim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004; 170:1108–13.

4). Gordon P, Sanders MH. Sleep.7: positive airway pressure therapy for obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2005; 60:68–75.
5). Varounis C, Katsi V, Kallikazaros IE, Tousoulis D, Stefanadis C, Parissis J, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: a systematic review and metaanalysis. Int J Cardiol. 2014; 175:195–8.

6). Lin MT, Lin HH, Lee PL, Weng PH, Lee CC, Lai TC, et al. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a metaanalysis. Sleep Breath. In press.
7). Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007; 132:1057–72.

8). Jokic R, Klimaszewski A, Sridhar G, Fitzpatrick MF. Continuous positive airway pressure requirement during the first month of treatment in patients with severe obstructive sleep apnea. Chest. 1998; 114:1061–9.

9). Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized Placebo-controlled Trial of Continuous Positive Airway Pressure on Blood Pressure in the Sleep Apnea-Hypopnea Syndrome. Am J Respir Crit Care Med. 2001; 163:344–8.

10). Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007; 30:711–9.

11). Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ. Factors impairing daytime performance in patients with sleep apnea/hypopnea syndrome. Arch Intern Med. 1992; 152:538–41.

12). Hack M, Davies RJ, Mullins R, Choi SJ, Ramdassingh-Dow S, Jenkinson C, et al. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax. 2000; 55:224–31.

13). Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med. 2011; 184:355–61.
14). Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000; 283:1829–36.
15). Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Pickering TG, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001; 154:50–9.
Go to : 
Table 1.
Definitions of equivalence margin for bootstrap method
| Variable | Equivalence margin |
|---|---|
| AHI, /hr | ±5 |
| PSQI | ±3 |
| CSAQLI | ±1 |
| SF-36 | ±15 |
| ESS | ±1 |
| SSS | ±3 |
| BSQ | ±2 |
| Blood pressure, mm Hg | Systolic: ±10 |
| Diastolic: ±5 |
Table 2.
Baseline characteristics of subjects
Table 3.
Analysis of AHI result after 4 weeks CPAP treatment using bootstrap method
| Variable | AHI, /hr | Mean (97.5% CI)* | Equivalence margin |
|---|---|---|---|
| Baseline | 53.2±24.0 | ||
| After 4 weeks standard CPAP | 3.0±3.7 | ||
| After 4 weeks newly developed CPAP | 2.5±2.0 | 2.5 (1.7∼3.5) | |
| Changes (After 4 weeks-baseline) | |||
| Change after standard CPAP | –50.2±24.7 | ||
| Change after newly developed CPAP | –50.7±24.2 | ||
| Difference of change (standard-newly) | 0.4±4.0 | 0.4 (−1.1∼2.5) | (−5∼5) |
Table 4.
Analysis of questionnaires associated with sleep after 4 weeks CPAP treatment using bootstrap method
| Variable | Result | Mean (95% CI)* | Equivalence margin |
|---|---|---|---|
| Quality of life | |||
| PSQI | |||
| Baseline | 7.5±2.4 | ||
| After 4 weeks standard CPAP | 6.1±3.5 | ||
| After 4 weeks newly developed CPAP | 5.1±3.0 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –1.4±2.3 | ||
| Change after newly developed CPAP | –2.4±2.2 | ||
| Difference of change (standard-newly) | 1.0±2.4 | 1.0 (−0.1∼2.1) | (−3∼3) |
| CSAQLI | |||
| Baseline | 4.51±0.74 | ||
| After 4 weeks standard CPAP | 5.29±0.92 | ||
| After 4 weeks newly developed CPAP | 5.24±0.79 | ||
| Changes (after 4 weeks-Baseline) | |||
| Change after Standard CPAP | 0.78±0.76 | ||
| Change after newly developed CPAP | 0.74±0.63 | ||
| Difference of change (standard-newly) | 0.05±0.77 | 0.05 (−0.35∼0.44) | (−1∼1) |
| SF-36 | |||
| Baseline | 48.5±15.5 | ||
| After 4 weeks standard CPAP | 54.8±17.2 | ||
| After 4 weeks newly developed CPAP | 53.8±16.5 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | 6.3±10.0 | ||
| Change after newly developed CPAP | 5.3±11.1 | ||
| Difference of change (standard-newly) | 1.0±13.6 | 1.0 (−5.4∼7.4) | (−15∼15) |
| Daytime sleepiness | |||
| ESS | |||
| Baseline | 10.5±4.2 | ||
| After 4 weeks standard CPAP | 8.1±2.8 | ||
| After 4 weeks newly developed CPAP | 8.1±3.9 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –2.4±4.8 | ||
| Change after newly developed CPAP | –2.4±4.4 | ||
| Difference of change (standard-newly) | –0.1±4.1 | –0.06 (−2.05∼1.50) | (−3∼3) |
| SSS | 2.7±0.8 | ||
| wBaseline | 2.4±1.0 | ||
| After 4 weeks standard CPAP | 2.1±0.7 | ||
| After 4 weeks newly developed CPAP | |||
| Changes (after 4 weeks-baseline) | –0.4±1.3 | ||
| Change after standard CPAP | –0.6±1.1 | ||
| Change after newly developed CPAP | 0.3±1.3 | ||
| Difference of change (standard-newly) | 0.2 (−0.3∼0.8) | (−1∼1) | |
| Snoring index | |||
| BSQ | |||
| Baseline | 6.4±2.1 | ||
| After 4 weeks standard CPAP | 2.1±2.3 | ||
| After 4 weeks newly developed CPAP | 3.9±3.0 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –4.3±3.0 | ||
| Change after newly developed CPAP | –2.5±2.9 | ||
| Difference of change (standard-newly) | –1.8±3.0 | –1.8 (−3.5†∼-0.1) | (−2∼2) |
Table 5.
Analysis of laboratory results associated with obstructive sleep apnea syndrome after 4 weeks CPAP treatment using bootstrap method
| Variable | Result | Mean (95% CI)* | Equivalence margin |
|---|---|---|---|
| Lipid profile, mg/dL | |||
| T-chol | |||
| Baseline | 193.7±37.8 | ||
| After 4 weeks standard CPAP | 190.3±29.6 | ||
| After 4 weeks newly developed CPAP | 192.55±37.3 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –3.4±20.9 | ||
| Change after newly developed CPAP | –1.1±20.5 | ||
| Difference of change (standard-newly) | –2.3±19.8 | –2.3 (−11.6∼7.0) | (−38∼38) |
| LDL-C | |||
| Baseline | 124.3±35.5 | ||
| After 4 weeks standard CPAP | 117.95±30.7 | ||
| After 4 weeks newly developed CPAP | 119.5±36 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –6.35±18.2 | ||
| Change after newly developed CPAP | –4.8±20.5 | ||
| Difference of change (standard-newly) | –1.55±26.5 | –1.6 (−11.5∼10.6) | (−52∼52) |
| TG | |||
| Baseline | 202.2±119 | ||
| After 4 weeks standard CPAP | 220.45±123.4 | ||
| After 4 weeks newly developed CPAP | 204.05±118.3 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | 18.25±62.5 | ||
| Change after newly developed CPAP | 1.85±105.9 | ||
| Difference of change (standard-newly) | 16.4±98.2 | 16.2 (−20.4∼58.5†) | (−52∼52) |
| Pulmonary function test, % of estimated volume | |||
| FVC | |||
| Baseline | 90.05±7.9 | ||
| After 4 weeks standard CPAP | 88.85±6.9 | ||
| After 4 weeks newly developed CPAP | 87.75±8.5 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –1.2±4.3 | ||
| Change after newly developed CPAP | –2.3±6 | ||
| Difference of change (standard-newly) | 1.1±3.6 | 1.1 (−0.6∼2.8) | (−8∼8) |
| FEV1 | |||
| Baseline | 91.45±12.2 | ||
| After 4 weeks standard CPAP | 89.75±10 | ||
| After 4 weeks newly developed CPAP | 88.1±12.1 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –1.7±6.3 | ||
| Change after newly developed CPAP | –3.35±9.9 | ||
| Difference of change (standard-newly) | 1.65±5.1 | 1.6 (−0.8∼3.7) | (−10∼10) |
| Cardiac marker, ng/mL | |||
| NT-proBNP | |||
| Baseline | 20.84±19.28 | ||
| After 4 weeks standard CPAP | 19.23±14.36 | ||
| After 4 weeks newly developed CPAP | 21.43±45.06 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –1.61±14.91 | ||
| Change after newly developed CPAP | 0.59±40.22 | ||
| Difference of change (standard-newly) | –2.21±40.22 | –2.5 (−22.3∼10.1) | (−78∼78) |
Table 6.
Analysis of physical examinations associated with obstructive sleep apnea syndrome after 4 weeks CPAP treatment using bootstrap method
| Variable | Result | Mean (95% CI)* | Equivalence margin |
|---|---|---|---|
| Blood pressure, mm Hg | |||
| Systolic | |||
| Baseline | 124.5±11.1 | ||
| After 4 weeks standard CPAP | 123.8±9.3 | ||
| After 4 weeks newly developed CPAP | 121.6±10.8 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –0.8±8.0 | ||
| Change after newly developed CPAP | –3.0±10.1 | ||
| Difference of change (standard-newly) | 2.2±6.4 | 2.2 (−0.8∼5.2) | (−10.0∼10.0) |
| Diastolic | |||
| Baseline | 83.2±11.4 | ||
| After 4 weeks standard CPAP | 79.3±9.0 | ||
| After 4 weeks newly developed CPAP | 76.3±7.2 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –3.9±8.5 | ||
| Change after newly developed CPAP | –6.9±9.4 | ||
| Difference of change (standard-newly) | 3.0±7.7 | 3.0 (−0.2∼6.2†) | (−5.0∼5.0) |
| Body measurement | |||
| Weight, Kg | |||
| Baseline | 76.5±12.3 | ||
| After 4 weeks standard CPAP | 76.5±12.0 | ||
| After 4 weeks newly developed CPAP | 76.1±12.2 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | 0.005±1.1 | ||
| Change after newly developed CPAP | –0.4±2.1 | ||
| Difference of change (standard-newly) | 0.4±1.9 | 0.4 (−0.5∼1.3) | (−4.0∼4.0) |
| Waist, cm | |||
| Baseline | 94.4±7.7 | ||
| After 4 weeks standard CPAP | 94.6±7.5 | ||
| After 4 weeks newly developed CPAP | 94.3±7.4 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | 0.2±2.6 | ||
| Change after newly developed CPAP | –0.04±1.2 | ||
| Difference of change (standard-newly) | 0.2±2.8 | 0.3 (−0.7∼1.6) | (−6.0∼6.0) |
| Neck-supine, cm | |||
| Baseline | 41.51±3.11 | ||
| After 4 weeks standard CPAP | 41.23±3.19 | ||
| After 4 weeks newly developed CPAP | 41.35±3.04 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –0.28±0.75 | ||
| Change after newly developed CPAP | –0.16±0.89 | ||
| Difference of change (standard-newly) | –0.13±0.70 | –0.13 (−0.42∼0.19) | (−1.38∼1.38) |
| Neck-sitting, cm | |||
| Baseline | 39.99±2.84 | ||
| After 4 weeks standard CPAP | 39.92±3.00 | ||
| After 4 weeks newly developed CPAP | 39.75±2.78 | ||
| Changes (after 4 weeks-baseline) | |||
| Change after standard CPAP | –0.07±0.69 | ||
| Change after newly developed CPAP | –0.24±0.45 | ||
| Difference of change (standard-newly) | 0.17±0.75 | 0.17 (−0.16∼0.48) | (−1.48∼1.48) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download