Abstract
A variety of intrinsic and extrinsic factors have been studied to explain the pathogenesis of rhinosinusitis. Recently biofilms are emerging as an important cause. Biofilms are highly organized structures composed of a protective extracellular matrix and bacterial colonies, and provide the means for bacterial survival and virulence. Biofilms are known to be associated with intractable cases of rhinosinusitis and antibiotic resistance. Patients diagnosed with biofilm-related rhinosinusitis tend to suffer more severe disease that those without biofilms. Biofilm severity can also influence the prognosis of rhinosinusitis. We present two cases of pseudomonas-induced macroscopic biofilms (bioballs) of the maxillary sinuses. These bioballs cause intractable chronic rhinosinusitis as well, but unlike traditional biofilms, they can be surgically removed by endoscopy, and thus have a better prognosis than traditional biofilms. This is the first report of visible biofilms (bioballs) found in the maxillary sinuses.
Go to : 
References
1). Chung SK. Biofilm. J Rhinol. 2005; 12:5–9.
2). Ferguson BJ, Stolz DB. Demonstration of biofilm in human bacterial chronic rhinosinusitis. Am J Rhinol. 2005; 19(5):452–7.

3). Sanclement JA, Webster P, Thomas J, Ramadan HH. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope. 2005; 115(4):578–82.

4). Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathology. Otolaryngol Head Neck Surg. 2003; 129(3):21–32.
5). Prince AA, Steiger JD, Khalid AN, Dogrhamji L, Reger C, Eau Claire S, et al. Prevalence of biofilm-forming bacteria in chronic rhinosinusitis. Am J Rhinol. 2008; 22(3):239–45.

6). Psaltis AJ, Ha KR, Beule AG, Tan LW, Wormald PJ. Confocal scanning laser microscopy evidence of biofilms0 in patients with chronic rhinosinusitis. Laryngoscope. 2007; 117(7):1302–6.
7). Christopher J., Paul S, Luanne HS, Garth DE. The role of biofilms in otolaryngologic infections. Curr Opin Otolaryngol Head Neck Surg. 2004; 12:185–90.
8). Christian JH, Rizwan M, Dale HR. Biofilm and persistent inflammation in endoscopic sinus surgery. Otolaryngology-Head and Neck Surgery. 2010; 143:697–8.
9). Cryer J, Schipor I, Perloff JR, et al. Evidence of bacterial biofilms in human chronic sinusitis. ORL J Otorhinolaryngol Relat Spec. 2004; 66:155–8.

10). Ferguson BJ, Stolz DB. Demonstration of biofilm in human bacterial chronic rhinosinusitis. Am J Rhinol. 2005; 19:452–7.

11). Sanderson AR, Leid JG, Hunsaker D. Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope. 2006; 116:1121–6.

12). Psaltis AJ, Weitzel EK, Ha KR, Wormald PJ. The effect of bacterial biofilms on post-sinus surgical outcomes. Am J Rhinol. 2008; 22:1–6.

13). Park YH, Kim EH, Seo ST, Lee SH, Kim JM, Koo BS. Formation of Biofilm in Patients with Chronic Otitis Media and Cholesteatoma. Korean J Otorhinolarygol. 2009; 52:124–8.

14). Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995; 49:711–45.

15). Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999; 284(5418):1318–22.

16). Vlastarakos PV, Nikolopoulos TP, Maragoudakis P, Tzagaroulakis A, Ferekidis E. Biofilms in ear, nose, and throat infections: How important are they? Laryngoscope. 2007; 117(4):669–73.

17). Ymele-Leki P, Ross JM. Erosion from Staphylococcus aureus biofilms grown under physiologically relevant fluid shear forces yields bacterial cells with reduced avidity to collagen. Appl Environ Microbiol. 2007; 73(6):1834–41.
18). Psaltis AJ, Ha KR, Beule AG, Tan LW, Wormald PJ. Confocal scanning laser microscopy evidence of biofilms in patients with chronic rhinosinusitis. Laryngoscope. 2007; 117(7):1302–6.

19). Sanclement JA, Webster P, Thomas J, Ramadan HH. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope. 2005; 115(4):578–82.

20). Choi JW, Seo ST, Kim SG, Kim YM, Rha KS. The Demonstration of Bacterial Biofilm and It's Impact on Postoperative Course in Patients with Chronic Rhinosinusitis. Korean J Otorhinolaryngol. 2010; 53:349–53.

21). Khosravi Y, Ling LC, Loke MF, Shailendra S, Prepageran N, Vadi-velu J. Determination of the biofilm formation capacity of bacterial pathogens associated with otorhinolaryngologic diseases in the Ma-laysian population. Eur Arch Otorhinolaryngol. 2014; 271:1227–33.

22). Hayes SM, Howlin R, Johnston DA, et al. Intracellular residency of Staphylococcus aureus within mast cells in nasal polyps: A novel observation. J Allergy Clin Immunol. 2015; 135(6):1648–51.

23). Biel MA, Pedigo L, Gibbs A, Loebel N. Photodynamic therapy of antibiotic-resistant biofilms in a maxillary sinus model. Int Forum Allergy Rhinol. 2013; 3:468–73.

24). Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012; 23:1–298.
25). Min YG, Jung HW. Postoperative care of paranasal sinusitis. J Rhinol. 1995; 2:83–8.
Go to : 
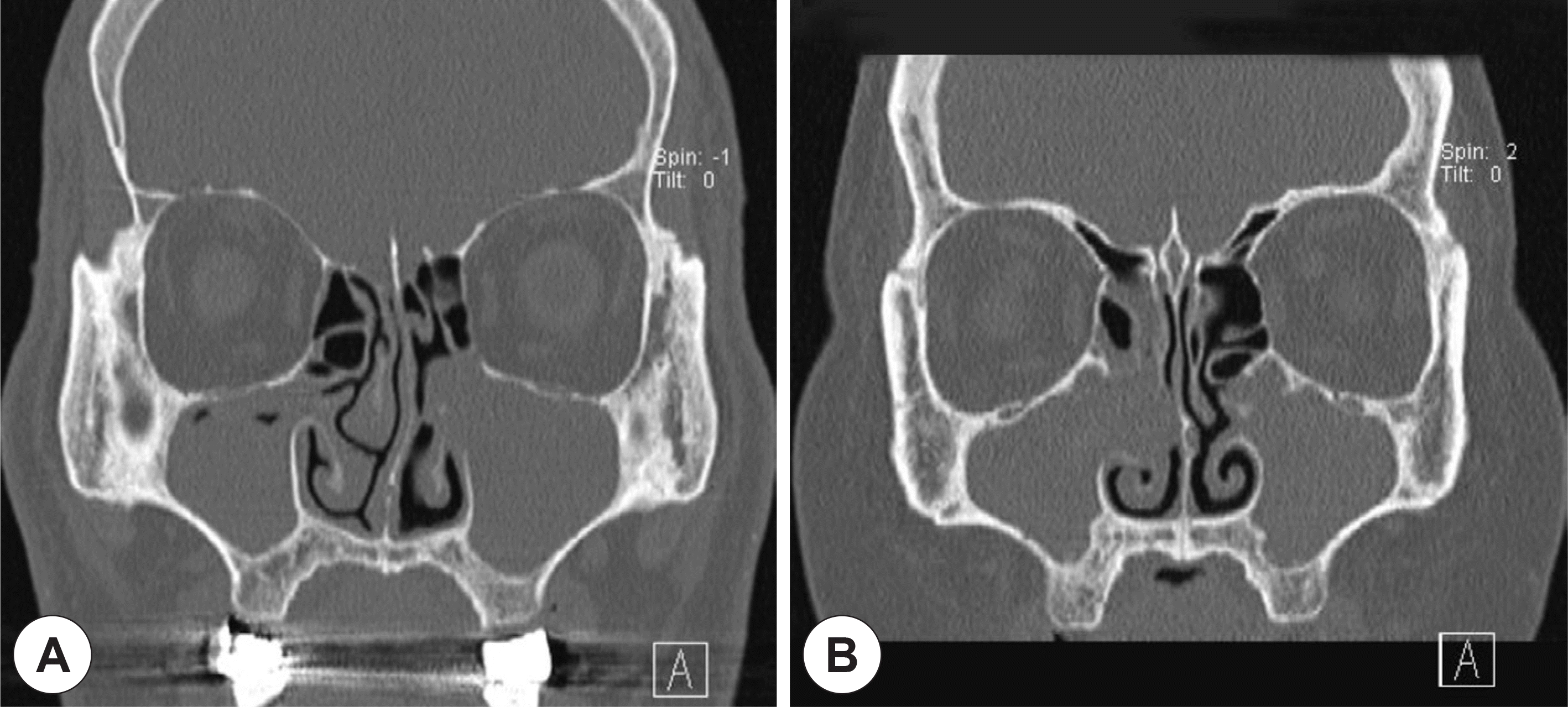 | Fig. 1.Preoperative paranasal CT scans of two cases of bioballs. Symptomatic maxillary sinusitis was located at the left side in case 1 (A) and the right side in case 2 (B). However, incidental maxillary sinusitis was also found at the opposite sides for each cases (bioballs). |
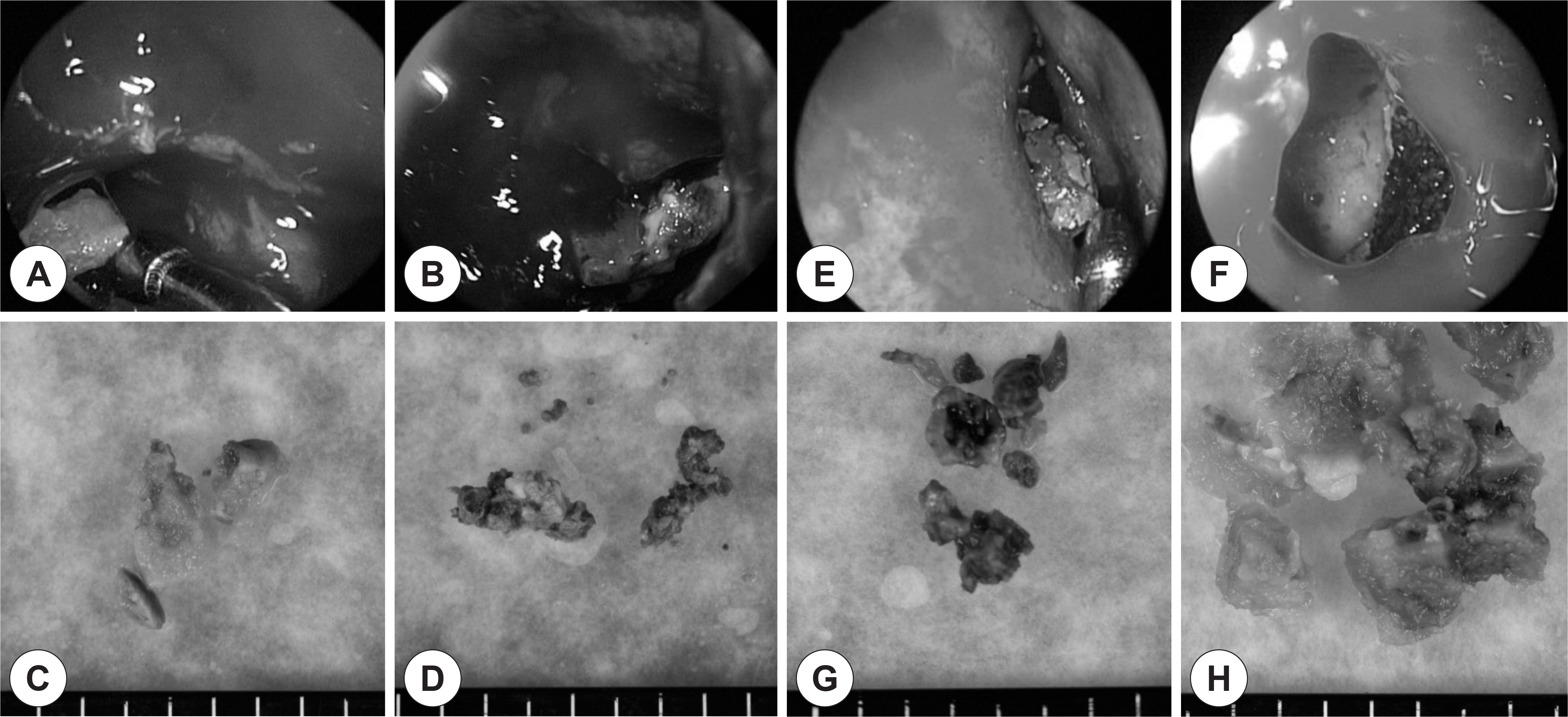 | Fig. 2.Intraoperative findings of bioballs (macroscopic biofilm) obtained from case 1 (A-D) and case 2 (E-H). Dark brown colored fungal balls were found at the left maxillary sinus of case 1 (B and D) and right maxillary sinus of case 2 (E and G). However, green or gray colored gel-like materials were found at the right maxillary sinus of case 1 (A and B) and the left maxillary sinuses of case 2 (F and H). |
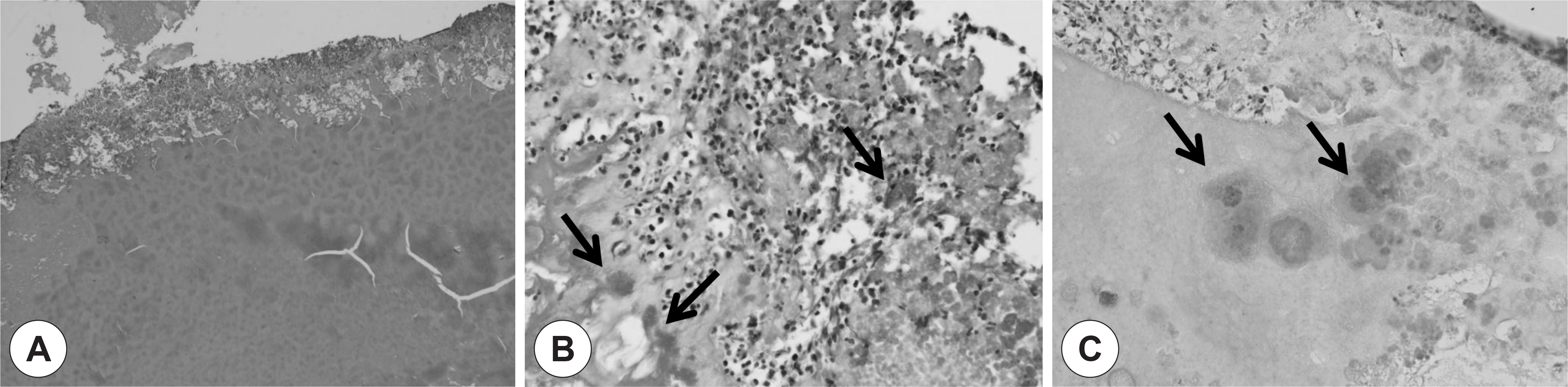 | Fig. 3.Microscopic findings of case 1. Greenish gel-like material (A, H-E stain, original magnification ×40) from the right maxillary sinus reveals a bioball consisting of thick acellular mucous material and peripherally arranged bacterial colonies (arrow) with acute inflammatory cell infiltrates (B, H-E stain, original magnification ×400). Most of bacterial colonies show gram negativity (C, Gram stain, original magnification ×400). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download