Abstract
Background and Objectives
Many kinds of inflammatory cells and cytokines are suggested to be related to the pathophysiology of chronic rhinosinusitis with nasal polyposis (CRSwNP), but not yet fully understood. The objectives of this study were to classify CRSwNP patients according to histologic features and to reveal the roles of IL-33 and IL-25, on the pathophysiology of CRSwNP. Materials and Method: CRSwNP patients (n=122) were divided into 3 groups according to the type of nasal polyp; eosinophilic polyp (EP, n=38), neutrophilic polyp (NeuP, n=15) and non-eosinophilic non-neutrophilic polyp (NENN, n=63) groups. Clinical features and the expressions of IL-25 and IL-33 were evaluated among the groups. Results: The EP group showed many clinical features that were different from the other groups: increased prevalence of asthma and olfactory dysfunction, increased percentage of blood eosinophils, increased E/M ratio, and poor postoperative outcomes such as recurrent polyposis and the need for frequent use of oral steroids. Numbers of IL-33 and IL-25 positive cells were significantly higher in the EP group compared with the other groups in the lamina propria (p=0.001). Conclusion: These results suggest that patients with an eosinophilic polyp are clinically and pathogenetically different from patients with other types of polyps, and IL-25 and IL-33 are associated with the pathogenesis of chronic rhinosinusitis with eosinophilic polyps.
References
1). Taylor MJ, Ponikau JU, Sherris DA, Kern EB. Detection of fungal organisms in eosinophilic mucin using a fluorescin-labelled chitin specific binding protein. Otolaryngol Head Neck Surg. 2002; 127(5):377–83.
2). Bachert C, Gevaert P, van Cauwenberge P. Staphylococcus aureus superantigens and airway disease. Curr Allergy Asthma Rep. 2002; 2:252–8.

3). Ikeda K, Shiozawa A, Ono N, Kusunoki T, Hirotsu M, Homma H, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013; 123:E1–9.

4). Yao T, Kojima Y, Koyanagi A, Yokoi H, Saito T, Kawano K, et al. Eo-taxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope. 2009; 119:1053–9.

5). Saitoh T, Kusunoki T, Yao T, Kawano K, Kojima Y, Miyahara K, et al. Role of interleukin-17A in the eosinophil accumulation and mucosal remodeling in chronic rhinosinusitis with nasal polyps associated with asthma. Int Arch Allergy Immunol. 2010; 151:8–16.

6). Suzuki H, Takahashi Y, Wataya H, Ikeda K, Nakabayashi S, Shimomura A, et al. Mechanism of neutrophil recruitment induced by IL-8 in chronic sinusitis. J Allergy Clin Immunol. 1996; 98:659–70.
7). Suzuki H, Ikeda K. Mode of action of longterm low-dose macrolide therapy for chronic sinusitis in the light of neutrophil recruitment. Curr Drug Targets Inflamm Allergy. 2002; 1:117–26.

8). Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010; 142:64–71.

9). Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005; 23:479–90.

10). Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA, et al. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000; 106:551–9.

12). Verri WA Jr, Guerrero AT, Fukada SY, Valerio DA, Cunha TM, Xu D, et al. IL-33 mediates antigen-induced cutaneous and articular hy-pernociception in mice. Proc Natl Acad Sci U S A. 2008; 105:2723–8.

13). Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 2006 203:. 1105–16.
14). Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001; 15:985–95.

15). Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011; 12:21–7.

16). Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012; 30:647–75.

17). Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010; 464:1367–70.

18). Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013; 188:432–9.

19). Baba S, Kondo K, Kanaya K, Suzukawa K, Ushio M, Urata S, et al. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2014; 124:E115–22.

20). Lildholdt T, Rundcrantz H, Bende M, Larsen K. Glucocorticoid treatment for nasal polyps. The use of topical budesonide, intramuscular betamethasone, and surgical treatment. Arch Otolaryngol Head Neck Surg. 1997; 123:595–600.
21). Lund VJ, MacKay IS. Staging in rhinosinusitis. Rhinology 1993;31 (4):. 183–4.
22). Sakuma Y, Ishitoya J, Komatsu M, Shiono O, Hirama M, Yamashita Y, et al. New clinical diagnostic criteria for eosinophilic chronic rhinosinusitis. Auris Nasus larynx. 2011; 38(5):583–8.

23). Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope. 1992; 102(57):1–18.
24). Settipane GA. Epidemiology of nasal polyp. Allergy Asthma Proc. 1996; 17:231–6.
25). Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008; 122:961–8.
26). Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS, et al. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007; 137:925–30.

27). Rinia AB, Kostamo K, Ebbens FA, van Drunen CM, Fokkens WJ. Nasal polyposis: a cellular-based approach to answering questions. Allergy. 2007; 62(4):348–58.
28). Sim JS, Kim SW, Lee KH, Cho JS. Significance of Peripheral Eosinophilia and Allergic Rhinitis in Chronic Rhinosinusitis. Korean J Otolaryngol. 2008; 51:234–9.
29). Bateman ND, Shahi A, Feeley KM, Woolford TJ. Activated eosinophils in nasal polyps: a comparison of asthmatic and non-asth-matic patients. Clin Otolaryngol. 2005; 30(3):221–5.

30). Dhong HJ, Ha BS, Rho HI, Bang EG, Lee CK. Histologic characteristics of chronic sinusitis with athma. Korean J Otolaryngol. 2002; 45:878–83.
31). Junichi I, Yasunori S, Mamoru T. Eosinophilic chronic rhinosinusitis in japan. Allergology International. 2010; 59:239–45.

32). Hoover GE, Newman LJ, Platts-Mills TA, Philips CD, Gross CW, Wheatley LM, et al. Chronic sinusitis: Risk factors for extensive disease. J Allergy Clin Immunol. 1997; 100:185–91.

33). Kamil A, Ghaffar O, Lavigne F, Taha R, Renzi PM, Hamid Q, et al. Comparison of inflammatory cell profile and Th2 cytokine expression in the ethmoid sinuses, maxillary sinuses, and turbinates of atopic subjects with chronic sinusitis. Otolaryngol Head Neck Surg. 1998; 118:804–9.

Fig. 2.
Classification of patients with chronic rhinosinusitis with nasal polyposis according to the histopathologic feature of the nasal polyp. A: Control. B: EP. C: NP. D: NENP. H&E stain (×400). Scale bar: 20 µm. EP: eosinophilic polyp. NP: neutrophilic polyp. NENP: non-eosinophilic & non-neutrophilic polyp.
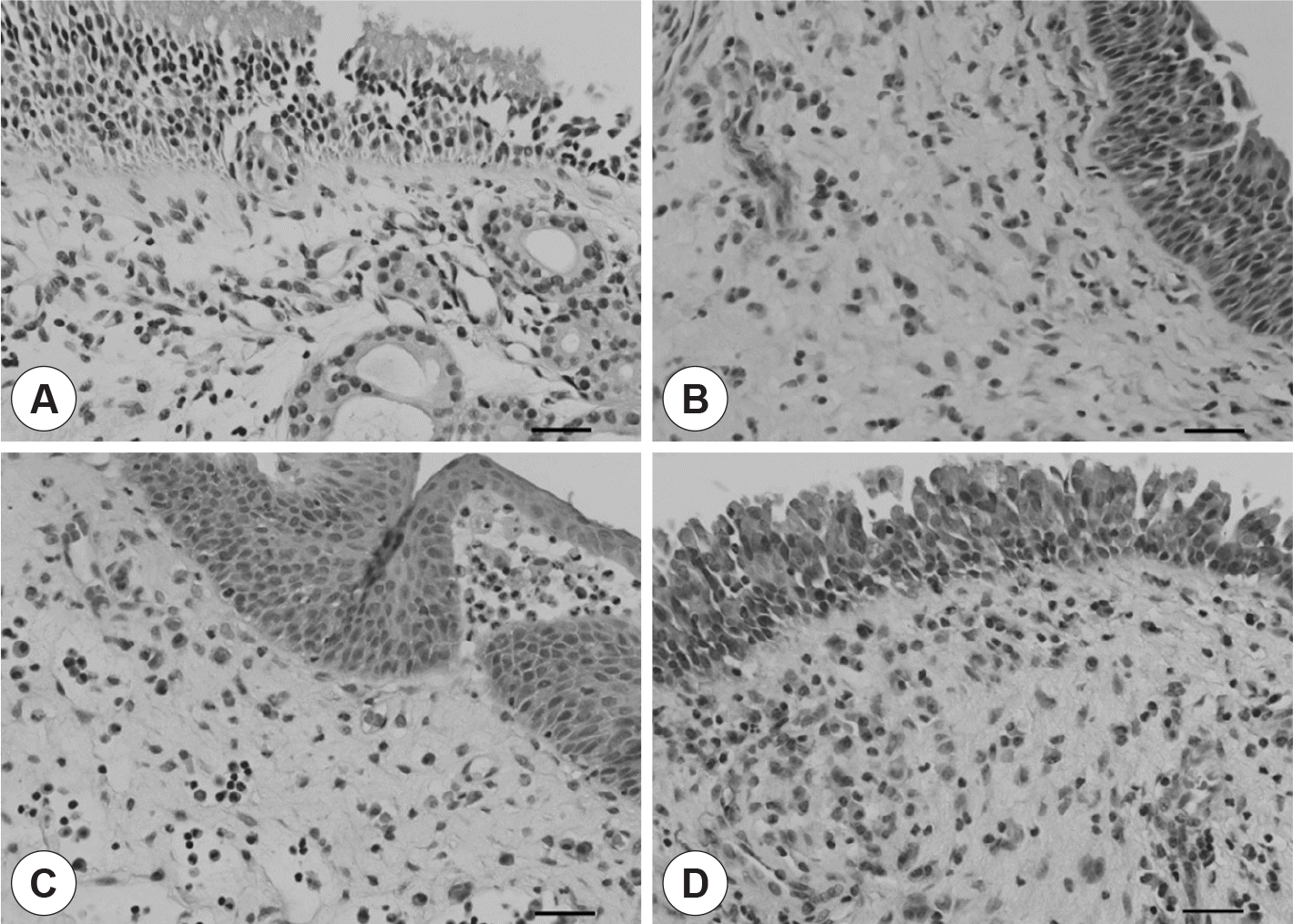
Fig. 3.
Correlation of blood eosinophil differential count and infiltrated eosinophil number of nasal polyps (A) and Relationship of infiltrated eosinophil number of nasal polyp and Lund-MacK-ay score (B) in patients with eosinophilic nasal polyp.
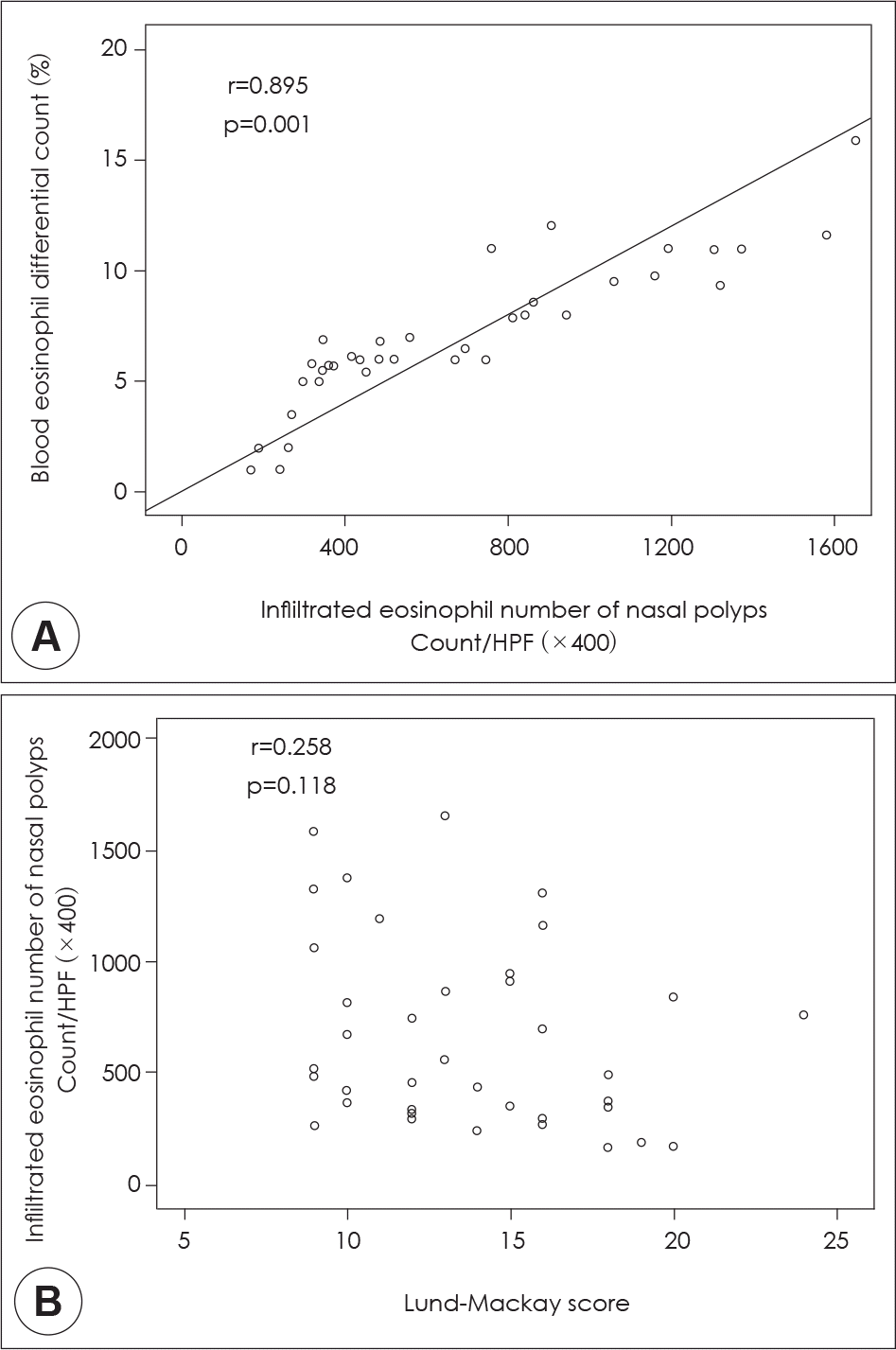
Fig. 4.
Expression patterns of IL-33 among the groups. Immunohistochemical stain was performed to detect of IL-33 expression in the tissues. A: Isotype control. B: Control group. C: EP. D: NP. E: NENP. IL-33 positive cells were count in the epithelium (F) and subepithelium (G). Immunohistochemical staining (×400). Scale bar: 20 µm. *: p<0.05 compared with other groups, †: p<0.05 compared with contol group. EP: eosinophilic polyp. NP: neutrophilic polyp. NENP: non-eosinophilic, & non-neutrophilic polyp.
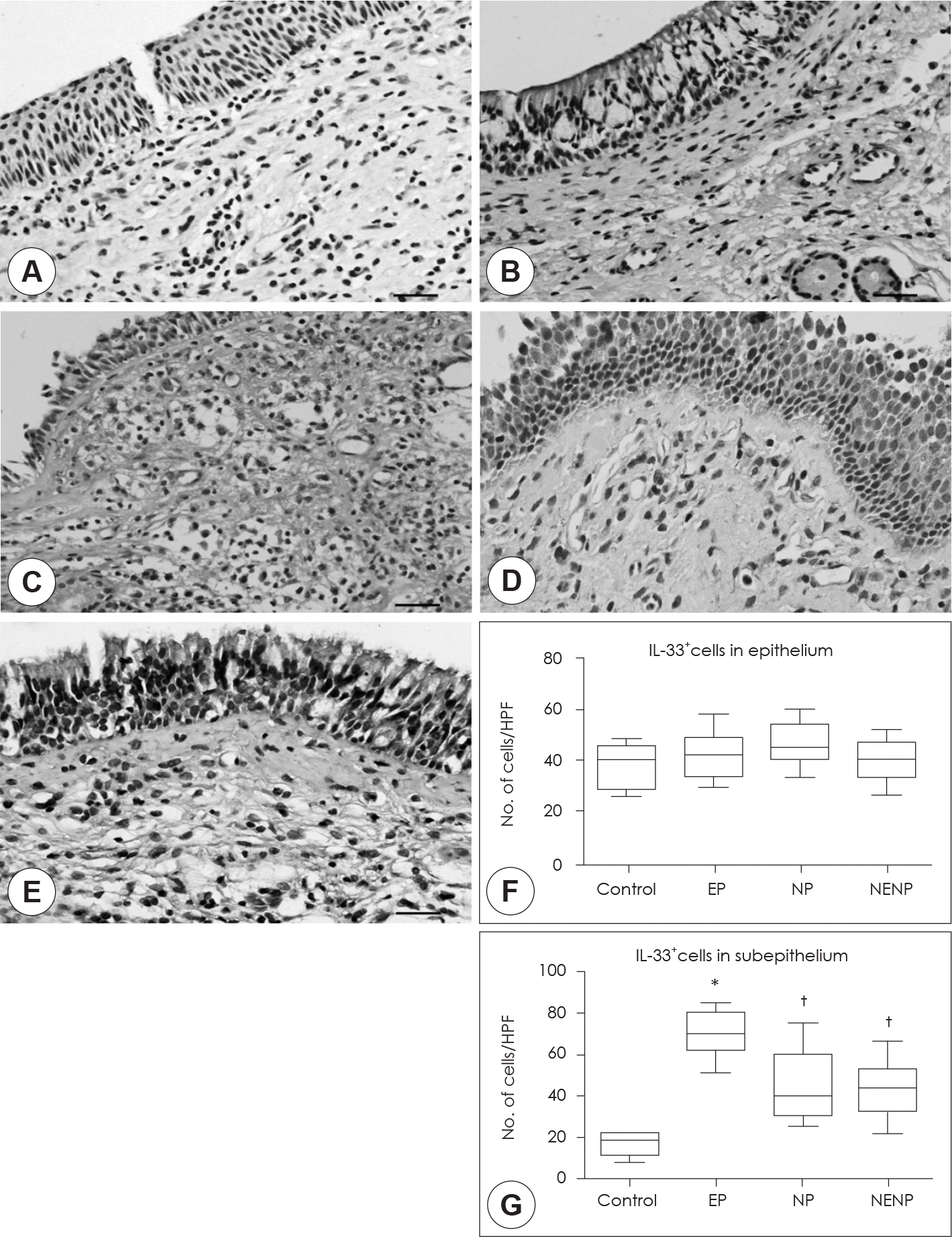
Fig. 5.
Expression patterns of IL-25 among the groups. Immunohistochemical stain was performed to detect of IL-25 expression in the tissues. A: Isotype control. B: Control group. C: EP. D: NP. E: NENP. IL-25 positive cells were count in the epithelium (F) and subepithelium (G). Immunohistochemical staining (×400). Scale bar: 20 µm. *: p<0.05 compared with other groups, †: p<0.05 compared with contol group. EP: eosinophilic polyp, NP: neutrophilic polyp, NENP: non-osinophilic & non-neutrophilic polyp.
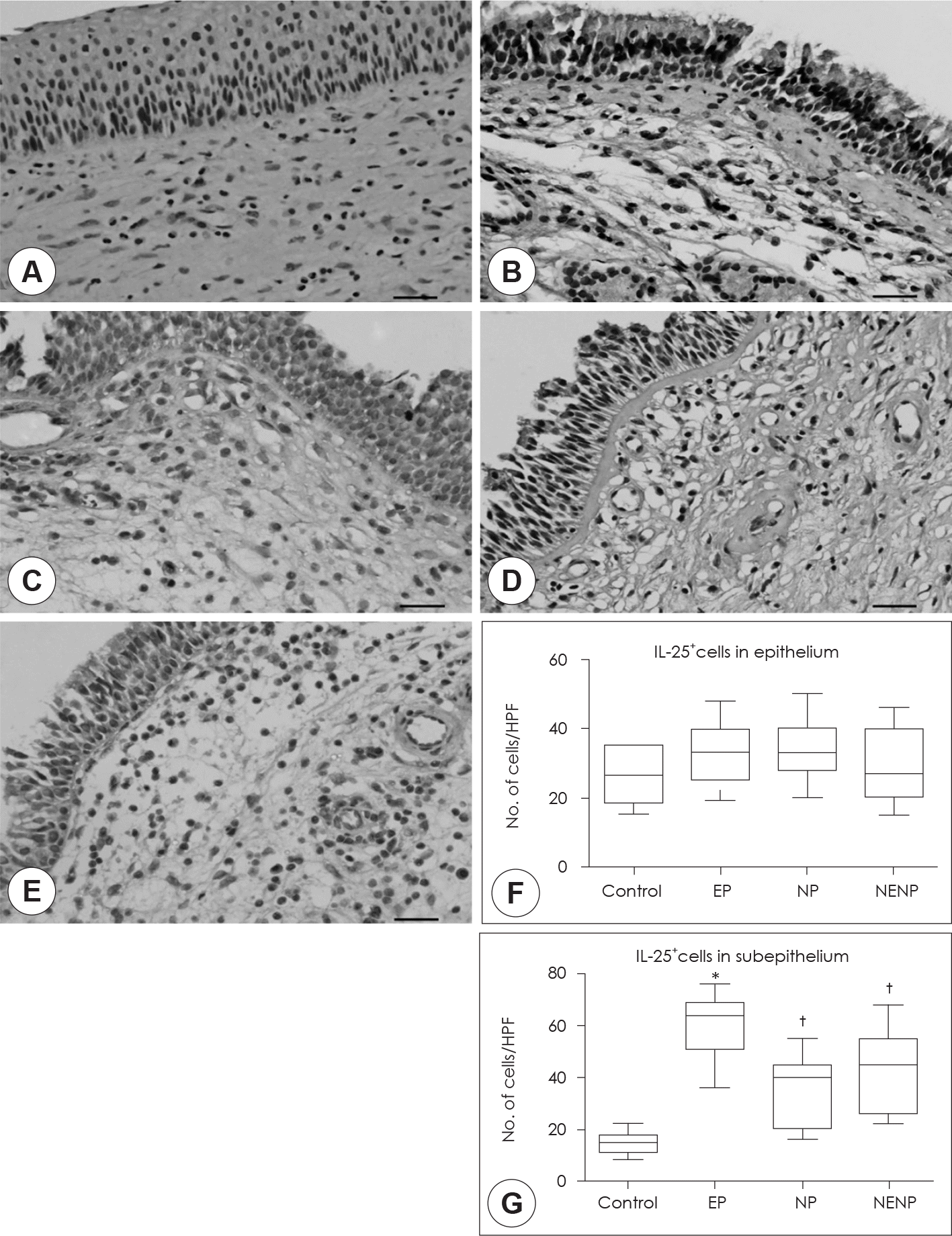
Table 1.
Patients characteristics
| Control (n=6) | EP (n=38) | NP (n=15) | NENP (n=63) | |
|---|---|---|---|---|
| Sex, male, n (%) | 4 (66.7) | 22 (57.8) | 12 (80) | 44 (69.8) |
| Age, mean± SD | 44.5±18.2 | 4.2±13.7 | 44.1±16.1 | 42.7±17.0 |
| Asthma, n (%) | 0 | 07 (18.5)* | 01 (6.7) | 03 (4.5) |
| Allergic rhinitis, n (%) | 0 | 05 (13.2) | 02 (13.3) | 07 (11.1) |
Table 2.
Clinical features of patients
| Control | EP | NP | NENP | |
|---|---|---|---|---|
| Olfactory dysfunction, n (%) | 0 | 32 (84.2)* | 8 (53.3) | 47 (69.1) |
| Blood eosinophil (%) | 1.67±1.4 | 6.8±3.3** | 2.1±1.5 | 3.2±1.7 |
| Lund-kennedy endoscopic score | 0 | 2.9±1.0 | 2.8±1.3 | 3.3±1.2 |
| Lund-mackay CT score | 0 | 13.7±3.8 | 12.5±3.9 | 15.9±5.2 |
| E/M ratio | ||||
| Total | – | 1.77±0.8** | 0.8±0.2 | 0.9±0.3 |
| Rt | – | 1.7±0.7* | 0.8±0.2 | 0.9±0.3 |
| Lt | – | 1.88±0.2** | 0.8±0.2 | 0.9±0.3 |
Table 3.
Post-operative outcomes of patients
| EP | NP | NENP | |
|---|---|---|---|
| Recurrent polyposis, n (%) | 11 (29)* | 2 (13.4) | 4 (5.9) |
| Oral steroid therapy, n (%) | 12 (31.6)* | 1 (6.7) | 4 (5.9) |
| Revision surgery, n (%) | – | – | 2 (3.2) |
Table 4.
Number of IL-33 and IL-25 positive cells in the epithelium and subepithelium among the groups. Mean±SD/HPF (×400)
| Control | EP | NP | NENP | ||
|---|---|---|---|---|---|
| IL-33 | Epithelium | 38.0±9.3 | 40.9±9.2 | 46.2±8.8 | 39.3±8.2 |
| Sub-epithelium | 19.0±7.6 | 70.0±10.8* | 45.1±15.9† | 43.6±13.5† | |
| IL-25 | Epithelium | 26.3±8.1 | 32.4±8.7 | 34.6±8.7 | 28.9±10.1 |
| Sub-epithelium | 14.5±4.6 | 60±11.5* | 37.3±13.0† | 43.5±14.6† |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download