Abstract
Ever since mankind has had blood, efforts to stop bleeding have never ceased and so numerous methods for hemostasis have been developed. In recent decades, minimally invasive surgical techniques have led patients to less-bleeding surgery but, hemostatic agents, devices and techniques still play an important role in medical side. A number of hemostatic agents and devices have been developed and they can be classified by their mechanism of action. That classification of the coagulants includes mechanisms with physical, caustic, bio-physical, biologic actions. Hemostatic devices are divided into categories such as dressings, glue, clips, electrocoagulations and so on. Based on the concept of minimally invasive surgical procedures, variously developed surgical techniques are divided by the number of ports used and auxiliary instruments. However, there are advantages and disadvantages to each of the hemostatic agents and minimally invasive methods, and the belief in the classical method also prevents the application of new hemostatic methods. The knowledge and understanding of the benefits and costs of these newly developed hemostatic methods will make it easier for medical personnel to manage patient's blood.
Hemostasis occurs when blood vessels are injured or ruptured and it is a series of responses for the body to stop bleeding without thrombus [1]. The rapid sequence of hemostatic processes can be divided into four steps and the whole sequence is shown in Fig. 1. Hemostasis is initiated with an arteriolar vasoconstriction process that the damaged blood vessels shrink to block blood from being spilled [2]. The second step after the arteriolar vasoconstriction is platelet aggregation and it is also called primary hemostasis. The third step is called secondary hemostasis or clot formation. The final step includes completed thrombus and antithrombotic events [34].
By the smooth muscle cells in vessel walls, vasoconstriction is the reflex of the blood vessels to damage. Controlled by vascular endothelium, smooth muscle cells release intravascular signals such as endothelin to control the contracting properties. The damaged vessels constrict to reduce the amount of blood outflow of the damaged vessel and to limit the volume of hemorrhage.
In this process regulated through thromboregulation, platelets are attached to the damaged endothelium to build a clot and degranulation. Von Willebrand factor (vWF), glycoprotein in plasma, activates plug formation. When platelets bump into the wounded endothelium cells, they change their shape, release granules and eventually become sticky. Activated platelets express glycoprotein receptors that have reciprocal action with other platelets and they undergo aggregation and adhesion process.
Cytoplasmic granules just as adenosine diphosphate (ADP), thromboxane A2 (TxA2) and serotonin are released from platelets. Adenosine diphosphate (ADP) is an attractant of platelets, serotonin is a vasoconstrictive modulator and thromboxane A2 is an assistant of vasoconstriction, platelet aggregation and degranulation. Being more chemicals released, this chain reaction makes more platelets sticky and creates platelet plugs. So the process continues in a positive feedback cycle [57].
Once platelets form platelet plugs, clotting factors are activated through a series of processes called coagulation casacades to activate fibrinogen to fibrin (Fig. 2). As a result, a fibrin mesh is created around the platelet plug to keep it in place, and this whole process is called secondary hemostasis. During this process some of the erythrocytes and leukocytes are entrapped in the meshwork which causes the primary platelet plug to become stronger and the resultant plug is called a thrombus or clot [3].
In this third step of hemostatic process, coagulation cascade is divided into two initial clotting pathways which convert fibrinogen into fibrin.
Fibrinolysis is an important process that staves off blood clots from burgeoning and becoming pathologic [2]. In the process of fibrinolysis, the products of coagulation, so called fibrin clots are broken down, plasmin, activated form of plasminogen cuts the fibrin meshwork, and circulating fragments of thrombus are eliminated by proteases or by liver and kidney [8].
Tissue plasminogen activator (t-PA) and urokinase are the agents that play an important role in converting plasminogen to activated form of plasmin, thus allowing fibrinolysis to occur and are inhibited by plasminogen activator inhibitor. Damaged endothelium of the blood vessels release t-PA into the blood stream very slowly and it causes the clot to break down. More tissue plasminogen activator and urokinase are produced by plasmin itself to generate plasmin [1].
Hemostatic agents in this category usually play a role before the first hemostatic phase, so, their usage has a broad spectrum from prevention to management in bleeding. These coagulants act as a physical barrier like tamponade or modulator against bleeding and they have been used in numerous surgical techniques for blood management because of their cost-effectiveness.
Physical methods like cooling of the damaged site will result in compression of the vessel and reduction of blood outflow. However, bouncing back of vasodilation can occur with tissue rewarming, so that, excessive temperature changes may also lead to tissue destruction [9].
Direct pressure on the bleeding site is a reflexive method for most clinicians in initial hemostasis. Pressure on the wound site shrinks capillaries, and allows platelet aggregration and initiation of the coagulation cascade. In the case of small wounds, complete hemostasis without further intervention can be achieved by direct pressure for one to several minutes [10].
A compound, combination of bee wax, paraffin, isopropyl palmitate, and a wax-softener, acts as a tampon for bleeding, particularly on bony surfaces [1112]. Although the material has high cost-effectiveness, since it has to be applied precisely to the bone, its usage is restricted in a number of dermatologic surgery procedures. Side effects of bone wax use incorporate granuloma formation by foreign body reactions, local contagions, inhibition of tissue regeneration and bone healing, and embolization to distant sites including the pulmonary circulation [131415].
Wang et al have shown in a mouse experiment that alkylene oxide copolymers can easily achieve hemostasis as well as bone wax without inhibiting bone growth [14]. The alkylene oxide copolymers, known as Ostene, are perceived as bone wax in the hands of practitioner, but it is hydrophilic and melts well in water [16]. Since it is biochemically unchanged in the human body and is erased from the human body safely, it is thought to be better than bone wax. Wellisz et al showed a comparison of Ostene with bone wax in rabbit experiments and found that Ostene showed stronger binding than bone wax [17]. While Ostene seems to carry out hemostasis without blunting bone healing or increasing incidence of infection, randomized controlled studies in human cases have not been reported.
Within this category, coagulants may induce demolition of tissue, protein coagulation and pigmentation. Clot formation takes place as a result and small vessels are sealed.
Frederic E. Mohs, inventor of Mohs micrographic surgery, introduced the usage of 45% zinc chloride paste to carry out tissue fixation and this hemostat was preferred in his surgical technique [18]. Zinc paste was found to be effective for the reduction of tumor progression and the amount of exudate associated with malignant wounds, and its use is effective for bleeding from malignant wounds [1920]. Zinc paste compounds are also used for curing skin cancer, genital bleeding in a patient with recurrent cervical cancer [1821].
Twenty percent ferric subsulfate solution, called Monsel's solution is regarded as a vessel sealant by protein precipitation as a consequence of the combined action of acidity and oxidization of its subsulfate group [2223]. It has effectiveness in blood coagulation at theinspection site of skin or mucosal biopsies and its low pH inhibits bacterial contamination [24]. With this medical effectiveness, Monsel's solution is easy to store at room temperature, is easy to handle, and is inexpensive. The efficacy of Monsel's solution is increased by its increased viscosity and the higher concentration of material allows better coagulation and control of a wound created during dermatologic surgery [22]. The complications of this solution are gray to brown pigmentation at the site of application, increased erythema, delayed reepithelialization, dermal fibrosis and so on [25].
Silver nitrate is a cost-effective and easy way to stop small bleedings and it doesn't require special storing precautions [26]. But it is shown that silver nitrate is not effective for large bleeding control such as hemorrhagic cystitis [27]. Eschar formed at the area of administration can prevent deep tissue damage, though silver nitrate results in collateral tissue damage that may delay tissue restoration [28].
Aluminium chloride is proposed to have action of hydrolysis to hydrogen chloride (HCl) and has an effect on coagulation in injured tissue, contraction of blood vessels, and activation of the extrinsic pathway of coagulation. In many practices, Monsel's solution has been replaced by aluminum chloride because this solution does not have a risk of pigmentation. Although aluminum chloride is an useful topical coagulant because of its cost effectiveness, painful paresthesias at the application site may occur, tissue irritation and its excessive application may bring about impeded epithelial regeneration of the wound site [25].
By contributing a 3D entangled structure for clotting, this group of hemostatic agents promote platelet aggregation and coagulation.
In 1945, Correl and Wise introduced Gelatin foam made from animal skin gelatin as hemostatic agent, and this purified gelatin solution can be applied as powder form, spongy foam, or film-like membrane. Gelatin is relatively inexpensive, easy to handle and with thrombin application, its hemostatic ability may be boosted [1328]. Gelfoam paste makes less contamination and relatively limited inhibition of bone restoration compared to bone wax, thus, it is a good substitute for suppression of bleeding from bony surfaces, like an incision wound at sternotomy [15]. Because Gelatin is more hygroscopic than oxidized cellulose and microfibrillar collagen agents and its volume can be enlarged twice, this agent creates a moist meshwork that promotes clot formation [10]. But this property of swelling can cause compressive complications, such as nerve compression or space narrowing.
Oxidized cellulose, made from botanical fiber by oxidization with nitrogen dioxide, was first introduced by Frantz in 1942 [1618]. Since it has loose architecture, this regenerated cellulose adheres more quickly to the nearby environment so that it works as a physical meshwork to stop bleeding. With antibacterial properties, oxidized celluloses are a valuable tool because they are relatively cheap, and easy to handle and to acquire. Foreign body reactions may occur when large amounts of oxidized cellulose remained in the damaged site, and this agent is not approved for direct application upon periosteum, perichondrium or graft bed [1022].
These microfibrillar collagens, made from bovine corium, provides a large surface to contact blood and it allows platelets to aggregate and form thrombus through the intrinsic coagulation pathway [2329]. Since their mechanisms depend on platelet activation, it has some inefficiency in patients who undergo severe thrombocytopenia, however, it seems to be useful in hemostasis of patients those who take profound heparinization with aspirin or heparin [24]. Complications including needless attachment, rejection for foreign bodies, or allergic reactions have been rarely addressed [25].
Hemostatic agents of this last category provoke a strong vasoconstriction or simulate the late responses of the coagulation cascade. Though not all, these hemostats on coagulation cascade are utilized in highly invasive procedures and are costly.
Epinephrine stimulates α-adrenoceptors that leads to excitatory effects and vasoconstriction, therefore, epinephrine used in cutaneous and mucosal surgery is considered to be effective in hemostasis [30]. With evidence that epinephrine induces platele aggregation, epinephrine also has a role as vasoconstrictive-hemostatic agent applied by injection at endoscopic surgery, tissue biopsies and surgeries in many fields [3132]. But postoperative rebound hyperemia increasing the risk of blood loss and reduced vasoconstrictive effect with lidocaine administration is known as significant precautions [30].
Thrombin is a natural enzyme that is formed from prothrombin through intrinsic and extrinsic pathways in the coagulation cascade and thrombin organizes the foundation of fibrin polymer by converting fibrinogen to fibrin [2]. With advantages of these natural physiologic mechanisms, thrombin avoids foreign body reaction or inflammation, therefore, the site of application is not irritated [10]. However, there are contraindications in the skin incision closure and thrombin may delay healing of wounds made from mechanical displacement by gelatin.
In the 1970s, a randomized controlled case study of traditional topical coagulants versus fibrin sealant demonstrated considerably faster bleeding control and diminished postoperative blood issues of fibrin sealant [33]. Since then, fibrin sealants, consist of 2 components that combine thrombin and fibrinogen, have been approved and used currently [34]. Fibrin sealants are useful in the patient who has been heparinized or who has coagulation impairment such as hemophilia or von Willebrand's disease, and they don't promote inflammation of tissue [353637]. Though, they are commonly used as helpful hemostats in cardiothoracic procedures, fibirin sealants are expensive and require skill from the operator [38].
Compared with fibrin sealants, platelet gel has a high concentration of platelets and growth factors that are crucial to commence and enhance tissue mending and regeneration for wound healing, and to provide hemostasis and adhesion [39]. These hemostatic properties and growth factors represented as platelet derived growth factor (PDGF) make platelet gel ideally utilized as a packing ingredient in endoscopic sinus surgery and other reconstructive surgery in orthopedic, cardiac, hepatic systems [40]. However, its expensive cost, dependence on practitioner experience and necessity for centrifuge drives platelet gel less attractive (Table 1).
Fibrin treatments were known as favorable methods in abdominal, cosmetic and cardiovascular surgery. Furthermore, dry and stable medium was needed for its use in practical settings [41]. In 1999, dry fibrin sealant dressing (DFSD) was introduced as gauze dressing that added freeze dried fibrinogen, calcium chloride, and thrombin to enhance coagulation at the injured site. Selected for American Red Cross program and US force, DFSD showed to be successful in animal studies focused on control of hemorrhage at liver, kidney, aorta and femoral artery [42]. Though problems about possible virus transmission were eliminated with blood donor screening, its expensive cost and need for strong protective packaging make DFSD less attractive [43].
Chitin and chitosan, poly-N-acetyl Glucosamine and its deacetylated form, both have hemostatic properties through the mechanisms of tissue adhesion, vasospasm and attraction of circulating blood cells [44]. Chitin dressing has effectiveness in treating small wounds by vasoconstriction and by recruitment of erythrocytes thrombocytes and coagulation factors, but in severe wounds, it has shown varying results compared with standard dressing [45].
With polycationic nature which gives it a natural antimicrobial property, chitosan dressing has an action of mechanical wound closure and adhesion on surrounding tissue, and its property and function makes chitosan dressings more effective than chitin dressings [46]. However, the effectiveness of chitosan dressing depends on how well the bandage is attached to the affected area, thus proper application is needed especially on wounds that are not flat. Chitin and chitosan dressings have advantages including lack of protein contamination and toxicity, easy storage and application, and disadvantages including high cost and variability of efficacy [44].
Zeolite, granular mineral mixture of aluminum, magnesium, silicon and sodium, stimulates hemostasis by making platelets and clotting factors (factor XII and XI in the intrinsic coagulation pathway) concentrated in wound sites through absorbing water molecules [47]. Proven in obstetric and general surgery for several years, zeolite agent is effective in controlling massive hemorrhage and is remarkably effective for low-pressure hemorrhage rather than high-pressure bleeding [4748]. Decreased effectiveness for high-pressure bleeding was reported and by enclosing it in a gauze pouch, zeolite agent can be administrated under relatively high-pressure without being washed away by the blood stream. Although zeolite is inexpensive, has antimicrobial properties and requires no special storage, it has some complications of thermal injury, scar formation and foreign body reaction [4448].
Cyanoacrylates, quickly polymerizing liquid monomers, create adhesion between tissue and physical blockade and prevent bleeding or fluid leakage [49]. With antibacterial properties, cyanoacrylates have been used to treat cutaneous wounds, gastric varices, and cerebrospinal fluid leakage [50]. The advantages of cyanoacrylates in tissue healing is known as more rapid application, faster healing time and waterproof barrier effect by tissue adhesion [51]. Cyanoacrylates can cause inflammatory reactions, fibrosis, neurotoxicity and delayed wound healing after application [10].
Consist of two ingredients of high-molecular weight polyethylene glycol and hydrogel matrix, polyethylene glycol hydrogel crosslink to each other and contact tissue. Right after that, this cross-linked meshwork of hydrogel forms a concrete layer against fluid leaking from tissue, and a defense wall for tissue regeneration and adhesion formation. Polyethylene glycol hydrogel agent is useful for bleeding suture of nonruptured aneurysms, preventing pericardial adhesions and treating pancreatic stumps or open wounds [52].
Because of its swelling property, polyethylene glycol hydrogel should not be used to the site where surrounding anatomic structures can be harmed by compression, instead, it could be a greatly effective hemostat for vascular, pulmonary and cardiac surgery in which extension is not an important problem [53]. Also this glue is not exothermic, does not have an inflammatory effect, and has no potential for bacterial infection.
BioGlue consists of dual cartilage of bovine serum albumin solution and glutaraldehyde solution that covalently crosslinks albumin, extracellular matrix and cell surfaces at the site of injury to form a tough scaffold [54]. Being used for cardiac surgery such as aortic dissecitons and aotic valve replacement, BioGlue can adhere to synthetic graft materials through mechanically interlocking with the graft matrix's interstices [55]. However, the use of BioGlue in young patients is not recommended, because problems resulting from the use in neonates and children are not reported [56].
The LigaSure vessel sealing system is a recently developed surgical device that uses mechanical pressure and an enhanced form of bipolar electrocoagulation to achieve permanent vessel wall fusion by denaturing the collagen and elastin in vessel walls and reforming them into a hemostatic seal [57]. Since its introduction, there are growing numbers of articles regarding the use of LigaSure in abdominal, thoracic, urological, and gynecological surgery with the main indication of dividing tissue [58].
Histologically, Ligasure demonstrated milder side thermal injury and faster healing process. Ligasure is clearly a safer and more efficient method of coagulation, whereas monopolar electrocautery could cause serious clinical and histological complications [59].
But the randomized controlled study in Japan suggested that LigaSure did not contribute to reduced operative time, intraoperative blood loss, or other adverse outcomes in open gastrectomy [57]. In addition, conversion rates and intraoperative and postoperative complication rates in laparoscopic adrenalectomy did not differ in both devices [60].
The patients undergoing head and neck surgery with the use of Harmonic devices had significant operative time reduction, less postoperative bleeding and less postoperative use of analgesics compared with hemostasis by electrocoagulation and ligature [61]. Compared to standard electrocautery devices, harmonic scalpel dissection presents significant advantages in decreasing intraoperative blood loss, postoperative drainage and wound complications in modified radical mastectomy without increasing operative time [62]. The meta-analysis comparing conventional surgery to Harmonic scalpel for gastrectomy and lymphadenectomy also demonstrates the advantages of Harmonic scalpel compared to conventional surgical techniques [63].
Recently developed energy based device, THUNDERBEAT appears to be equally safe and effective compared to other energy sources, such as standard electrosurgery, ultrasonic coagulating shears and electrothermal bipolar vessel sealers in patients those who undergo laparoscopic colorectal resection [64]. Compared with the other instruments, THUNDERBEAT has a faster dissection speed, acceptable thermal spread, and similar bursting pressure. Thus, this new surgical device is a charming and a safe alternative for tissue dissection during surgery, cutting and coagulation [65].
In the early 1990s, research on minimally invasive surgical methods was actively reported. There are two representative innovative technologies, one of which is the transluminal approach through the openings in the human body and the other is the transumbilical approach through the navel. The transluminal approach is an approach that inserts an endoscopic instrument through the mouth, anus, vagina, urethra, and the like. Commonly used single laparoscopic surgery is a transumbilical approach using an embryologic orifice that was open at the time of embryo [66].
The inferior epigastric artery is generally known to originate from the external iliac artery between inguinal ligament and is up to 6 cm in height, but it is not uncommon to find other routes. There is evidence that if the inferior epigastric artery starts below the inguinal ligament, it also branches off from the obturator artery, the femoral artery, and the external iliac artery, and it is also reported that there are many variations in branches of the inferior epigastric artery [67].
Epstein et al. considered the abdominal midline as an area that is free from blood vessels and also regarded the area from the imaginary line connecting the midline of the abdomen two thirds the way to the anterior superior iliac spines (ASIS) as a safety zone from large vessels. It was reported that insertion of a trocar through this site can avoid the injury of blood vessels. Raje et al. noted that the Inferior epigastric artery injury was rare in a laparoscopic approach to bilateral inguinal hernia surgery, but almost all reported cases occurring by trocar injuries [68].
Quint et al. attempted to find the superior abdominal vessels by transillumination prior to insertion of the trocar and 64% of the 103 patients were able to identify the vessel. Through the transilluminating method, secondary vessels in up to 90% of those 5 cm from the abdominal midline and 51% of those 8 cm from the midline could be found. Finally, the authors concluded that mapping the lower abdominal wall arteries through the transilluminating method cannot be a decisive method [69].
It is also referred to as scarless surgery, single-port laparoscopy, single-port umbilical surgery, natural orifice transumbilical surgery, laparoendoscopic single-site surgery, or embryonic natural orifice transumbilical endoscopic surgery depending on the nature and form of the operation. In Korea, since the 1970s, the yoon's ring has been used or tubal ligation has been performed through single-navel incision in obstetrics and gynecology [70].
In 1991, Pelosi et al. performed single-port laparoscopic hysterectomy and bilateral salpingo-oophorectomy, and since then single-port laparoscopic surgery has become possible with laparoscopic surgery and laparotomy in gynecology [71]. Single-port laparoscopic surgery has advantages of less scarring compared to conventional multi-port total laparoscopic hysterectomy (TLH), as well as the advantages of better surgical outcomes such as postoperative pain and trauma bleeding [72].
But, surgeons are reluctant to perform single-port laparoscopic surgery due to various problems that arise because of the operational limitations such as fencings (interference between instruments or endoscopes). In addition, an unstable camera platform with a single hole could result in lower surgical accuracy and longer operating time compared to conventional laparoscopic surgery. To overcome these inconveniences, the laparoscopic apparatus used an angled instrument and used a mechanism to bend the instrument. However, there is also the problem that these new instruments can be used freely after being trained well in order to use them at a higher price than existing equipment.
There is a method of inserting an assist device into the lower abdomen with single-port laparoscopy at undulatory state. In this procedure, 5 mm needle-form tube is inserted into the pubic bone except for the port inserted in the navel, and in the case of using a modified single-port laparoscopic surgical procedure, a minilaparoscopic grasper is applied to the same pubic bone without skin incision. After the absence of blood vessels in the pubic bone was checked, a mini-laparoscopic grasper is inserted by twisting through the small incision which is made on the skin. Then the laparoscopic observation of the tip into the abdominal cavity is performed and the end of the needle in the abdominal cavity is pulled out of the abdomen. Through the 5mm tube pierced in the navel, the instrument to be used is combined at the end of the needle and is pushed back into the abdominal cavity. After removing the needle pushed to the lower abdomen, the handle of the instrument is attached to the tip of the inserted instrument that entered the tube of the navel.
In addition to solving the technical difficulties caused by single-port laparoscopic surgery, it can be used at low cost, and it can reduce the side effects such as vascular damage and improve the satisfaction of the patients receiving the treatment. Because of this advantage, it can be used universally performed in the field of minimal invasive surgery.
In addition to the well-known hemostatic agents, devices and techniques mentioned above, numerous hemostatic methods are developed and released at different times. These rapid advances in patient blood management have been steadily introduced through several review articles, and numerous papers are described at different foci based on different clinical experiences.
As with these different foci, the way in which medicines and instruments are categorized is not clearly defined, so that the medicines and instruments are categorized in different ways in different articles. Of course, some sort of classification is made according to the mechanism of agents and application method of them. This review also classifies hemostats and devices considering the common parts of the methods used in the existing review papers.
Therefore, it is necessary for the clinician to judge based on various papers and experiences, as well as a quick and periodic update of the relevant information about the mechanism, effects and side effects of each agent, apparatus and technique.
Figures and Tables
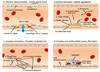 | Fig. 1(A) Arteriolar vasoconstriction occurs immediately by the reflex mechanism of the nervous system right after vascular injury, which can be enhanced by endothelin, a potent vasoconstrictor released from the endothelial cells constituting the vessel wall. (B) Platelets bind to the von Willebrand factor and attach to the extracellular matrix at the site of injury, after that, they change their appearance and promote further recruitment and aggregation of platelets by releasing granules such as ADP and Tx A2. (C) Tissue factor released from vascular endothelial cells expresses the platelet phospholipid complex. Through the coagulation cascade, they eventually activate thrombin and ultimately make the fibrin polymer to form thrombus. (D) During this period, the platelet plug contains trapped neutrophils and RBCs in the blood vessels, showing permanent plugs and preventing further bleeding. In the absence of vascular injury or complete thrombus formation, the endothelial cells secrete t-PA and thrombomodulin, which inhibit platelet adhesion and aggregation, to exert antithrombotic effects that lead limitation of hemostasis. |
 | Fig. 2The main role of extrinsic pathway that follows green arrows is generation of thrombin burst and thrombin burst is processed by thrombin, the most important coagulation factor of the coagulation cascade. It starts with tissue factors from damaged tissue and coagulation factor VII that moves around in blood plasma in a higher concentration than any other factors. The intrinsic pathway of red lines is activated by the primary complex formation on collagen with prekallikrein, high-molecular-weight kininogen (HMWK) and coagulation factor XII, known as Hageman factor. This intrinsic pathway seems to have association with inflammation and innate immunity. These two courses of coagulation pathway are the intrinsic pathway and the extrinsic pathway which both are concluded in the same common pathway along the black line to form cross-linked fibrin fibers from fibrinogen. SPCA, Serum prothrombin conversion accelerator; AHF, antihemophilic factor; AHG, antihemophilic globulin; PTC, plasma thromboplastin component; PTA, plasma thromboplastin antecedent. |
References
1. Robbins SL, Kumar V, Cotran RS. Robbins and Cotran pathologic basis of disease. 8th ed. Philadelphia, PA: Saunders/Elsevier;2010.
2. Hall JE, Guyton AC. Guyton and Hall textbook of medical physiology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier;2011.
3. Marieb EN, Hoehn K. Human anatomy & physiology. 8th ed. San Francisco: Benjamin Cummings;2010.
5. Porrett PM, Atluri P, Karakousis GC, Roses RE, Drebin JA. The surgical review: an integrated basic and clinical science study guide. 4th ed.
6. Zdanowicz MM. Essentials of pathophysiology for pharmacy. Boca Raton: CRC Press;2003.
7. Li Z, Delaney MK, O'Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010; 30:2341–2349.

8. Long AT, Kenne E, Jung R, Fuchs TA, Renne T. Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost. 2016; 14:427–437.

9. Bahn SL, Mursch PI. The effects of cold on hemostasis. Oral Surg Oral Med Oral Pathol. 1980; 49:294–300.

10. Larson PO. Topical hemostatic agents for dermatologic surgery. J Dermatol Surg Oncol. 1988; 14:623–632.
11. Tan TC, Black PM. Sir Victor Horsley (1857-1916): pioneer of neurological surgery. Neurosurgery. 2002; 50:607–611. discussion 11-2.

12. Savva A, Taylor MJ, Beatty CW. Management of cerebrospinal fluid leaks involving the temporal bone: report on 92 patients. Laryngoscope. 2003; 113:50–56.

13. Schonauer C, Tessitore E, Barbagallo G, Albanese V, Moraci A. The use of local agents: bone wax, gelatin, collagen, oxidized cellulose. Eur Spine J. 2004; 13:Suppl 1. S89–S96.

14. Wang MY, Armstrong JK, Fisher TC, Meiselman HJ, McComb GJ, Levy ML. A new, pluronic-based, bone hemostatic agent that does not impair osteogenesis. Neurosurgery. 2001; 49:962–967. discussion 8.

15. Wilkinson HA, Baker S, Rosenfeld S. Gelfoam paste in experimental laminectomy and cranial trephination: hemostasis and bone healing. J Neurosurg. 1981; 54:664–667.
16. Landry JR, Kanat IO. Considerations in topical hemostasis. J Am Podiatr Med Assoc. 1985; 75:581–585.

17. Wellisz T, An YH, Wen X, Kang Q, Hill CM, Armstrong JK. Infection rates and healing using bone wax and a soluble polymer material. Clin Orthop Relat Res. 2008; 466:481–486.

18. Frantz VK. Absorbable Cotton, Paper and Gauze: (Oxidized Cellulose). Ann Surg. 1943; 118:116–126.
19. Fukuyama Y, Kawarai S, Tezuka T, Kawabata A, Maruo T. The palliative efficacy of modified Mohs paste for controlling canine and feline malignant skin wounds. Vet Q. 2016; 36:176–182.

20. Kakimoto M, Tokita H, Okamura T, Yoshino K. A chemical hemostatic technique for bleeding from malignant wounds. J Palliat Med. 2010; 13:11–13.

21. Yanazume S, Douzono H, Yanazume Y, Iio K, Douchi T. New hemostatic method using Mohs' paste for fatal genital bleeding in advanced cervical cancer. Gynecol Oncol Case Rep. 2013; 4:47–49.

22. Scher KS, Coil JA, Jr . Effects of oxidized cellulose and microfibrillar collagen on infection. Surgery. 1982; 91:301–304.
23. Wagner WR, Pachence JM, Ristich J, Johnson PC. Comparative in vitro analysis of topical hemostatic agents. J Surg Res. 1996; 66:100–108.
24. Abbott WM, Austen WG. The effectiveness and mechanism of collagen-induced topical hemostasis. Surgery. 1975; 78:723–729.
25. Alexander JM, Rabinowitz JL. Microfibrillar collagen (Avitene) as a hemostatic agent in experimental oral wounds. J Oral Surg. 1978; 36:202–205.
26. Uluyol S. Effects of silver nitrate cauterization on middle turbinate synechia after endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2017; 157:515–518.

27. Montgomery BD, Boorjian SA, Ziegelmann MJ, Joyce DD, Linder BJ. Intravesical silver nitrate for refractory hemorrhagic cystitis. Turk J Urol. 2016; 42:197–201.

30. Naftalin LW, Yagiela JA. Vasoconstrictors: indications and precautions. Dent Clin North Am. 2002; 46:733–746. ix

31. Spalding A, Vaitkevicius H, Dill S, MacKenzie S, Schmaier A, Lockette W. Mechanism of epinephrine-induced platelet aggregation. Hypertension. 1998; 31:603–607.

32. Anschuetz L, Bonali M, Guarino P, Fabbri FB, Alicandri-Ciufelli M, Villari D, et al. Management of bleeding in exclusive endoscopic ear surgery: pilot clinical experience. Otolaryngol Head Neck Surg. 2017; 700–706.

33. Rousou J, Levitsky S, Gonzalez-Lavin L, Cosgrove D, Magilligan D, Weldon C, et al. Randomized clinical trial of fibrin sealant in patients undergoing resternotomy or reoperation after cardiac operations. A multicenter study. J Thorac Cardiovasc Surg. 1989; 97:194–203.

34. Spotnitz WD. Fibrin Sealant: the only approved hemostat, sealant, and adhesive-a laboratory and clinical perspective. ISRN Surg. 2014; 2014:203943.

35. Martinowitz U, Schulman S. Fibrin sealant in surgery of patients with a hemorrhagic diathesis. Thromb Haemost. 1995; 74:486–492.

36. Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001; 108:1713–1726.

38. Thompson DF, Letassy NA, Thompson GD. Fibrin glue: a review of its preparation, efficacy, and adverse effects as a topical hemostat. Drug Intell Clin Pharm. 1988; 22:946–952.

39. Bhanot S, Alex JC. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002; 18:27–33.

40. Pomerantz J, Dutton JM. Platelet gel for endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2005; 114:699–704.

41. Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010; 251:217–228.
42. Pusateri AE, Kheirabadi BS, Delgado AV, Doyle JW, Kanellos J, Uscilowicz JM, et al. Structural design of the dry fibrin sealant dressing and its impact on the hemostatic efficacy of the product. J Biomed Mater Res B Appl Biomater. 2004; 70:114–121.

43. Neuffer MC, McDivitt J, Rose D, King K, Cloonan CC, Vayer JS. Hemostatic dressings for the first responder: a review. Mil Med. 2004; 169:716–720.

44. Alam HB, Burris D, DaCorta JA, Rhee P. Hemorrhage control in the battlefield: role of new hemostatic agents. Mil Med. 2005; 170:63–69.

45. Kulling D, Vournakis JN, Woo S, Demcheva MV, Tagge DU, Rios G, et al. Endoscopic injection of bleeding esophageal varices with a poly-N-acetyl glucosamine gel formulation in the canine portal hypertension model. Gastrointest Endosc. 1999; 49:764–771.

46. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003; 4:1457–1465.

47. Noh JH, Lee JW, Nam YJ, Choi KY. Is intraoperative use of quikclot combat gauze effective for hemostasis after total knee arthroplasty? Clin Orthop Surg. 2017; 9:43–49.

48. Rhee P, Brown C, Martin M, Salim A, Plurad D, Green D, et al. QuikClot use in trauma for hemorrhage control: case series of 103 documented uses. J Trauma. 2008; 64:1093–1099.

50. Lo GH, Lai KH, Cheng JS, Chen MH, Chiang HT. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001; 33:1060–1064.

51. Spotnitz WD, Burks S. Hemostats, sealants, and adhesives III: a new update as well as cost and regulatory considerations for components of the surgical toolbox. Transfusion. 2012; 52:2243–2255.

52. Torchiana DF. Polyethylene glycol based synthetic sealants: potential uses in cardiac surgery. J Card Surg. 2003; 18:504–506.
53. Saunders MM, Baxter ZC, Abou-Elella A, Kunselman AR, Trussell JC. BioGlue and Dermabond save time, leak less, and are not mechanically inferior to two-layer and modified one-layer vasovasostomy. Fertil Steril. 2009; 91:560–565.

54. Biggs G, Hafron J, Feliciano J, Hoenig DM. Treatment of splenic injury during laparoscopic nephrectomy with BioGlue, a surgical adhesive. Urology. 2005; 66:882.

55. Luk A, David TE, Butany J. Complications of Bioglue postsurgery for aortic dissections and aortic valve replacement. J Clin Pathol. 2012; 65:1008–1012.

56. Passage J, Jalali H, Tam RK, Harrocks S, O'Brien MF. BioGlue Surgical Adhesive--an appraisal of its indications in cardiac surgery. Ann Thorac Surg. 2002; 74:432–437.

57. Fujita J, Takiguchi S, Nishikawa K, Kimura Y, Imamura H, Tamura S, et al. Randomized controlled trial of the LigaSure vessel sealing system versus conventional open gastrectomy for gastric cancer. Surg Today. 2014; 44:1723–1729.

58. Santini M, Fiorelli A, Messina G, Mazzella A, Accardo M. The feasibility of ligasure to create intestinal anastomosis: results of ex vivo study. Surg Innov. 2015; 22:266–273.

59. Diamantis T, Kontos M, Arvelakis A, Syroukis S, Koronarchis D, Papalois A, et al. Comparison of monopolar electrocoagulation, bipolar electrocoagulation, Ultracision, and Ligasure. Surg Today. 2006; 36:908–913.

60. Solaini L, Arru L, Merigo G, Tomasoni M, Gheza F, Tiberio GA. Advanced sealing and dissecting devices in laparoscopic adrenal surgery. Jsls. 2013; 17:622–626.

61. Prgomet D, Janjanin S, Bilic M, Prstacic R, Kovac L, Rudes M, et al. A prospective observational study of 363 cases operated with three different harmonic scalpels. Eur Arch Otorhinolaryngol. 2009; 266:1965–1970.

62. Huang J, Yu Y, Wei C, Qin Q, Mo Q, Yang W. Harmonic scalpel versus electrocautery dissection in modified radical mastectomy for breast cancer: a meta-analysis. PLoS One. 2015; 10:142–271.

63. Cheng H, Hsiao CW, Clymer JW, Schwiers ML, Tibensky BN, Patel L, et al. Gastrectomy and D2 lymphadenectomy for gastric cancer: a meta-analysis comparing the harmonic scalpel to conventional techniques. Int J Surg Oncol. 2015; 2015:397260.

64. Allaix ME, Arezzo A, Giraudo G, Arolfo S, Mistrangelo M, Morino M. The thunderbeat and other energy devices in laparoscopic colorectal resections: analysis of outcomes and costs. J Laparoendosc Adv Surg Tech A. 2016.

65. Milsom J, Trencheva K, Monette S, Pavoor R, Shukla P, Ma J, et al. Evaluation of the safety, efficacy, and versatility of a new surgical energy device (THUNDERBEAT) in comparison with Harmonic ACE, LigaSure V, and EnSeal devices in a porcine model. J Laparoendosc Adv Surg Tech A. 2012; 22:378–386.

66. Podolsky ER, Rottman SJ, Poblete H, King SA, Curcillo PG. Single port access (SPA) cholecystectomy: a completely transumbilical approach. J Laparoendosc Adv Surg Tech A. 2009; 19:219–222.

67. Epstein J, Arora A, Ellis H. Surface anatomy of the inferior epigastric artery in relation to laparoscopic injury. Clin Anat. 2004; 17:400–408.

68. Sriprasad S, Yu DF, Muir GH, Poulsen J, Sidhu PS. Positional anatomy of vessels that may be damaged at laparoscopy: new access criteria based on CT and ultrasonography to avoid vascular injury. J Endourol. 2006; 20:498–503.

69. Quint EH, Wang FL, Hurd WW. Laparoscopic transillumination for the location of anterior abdominal wall blood vessels. J Laparoendosc Surg. 1996; 6:167–169.

70. Quinones GR, Alvarado DA, Ley Ch E. [Tubal ligation using Yoon's ring]. Ginecol Obstet Mex. 1976; 40:127–136.
71. Pelosi MA, Pelosi MA, 3rd . Laparoscopic hysterectomy with bilateral salpingo-oophorectomy using a single umbilical puncture. N J Med. 1991; 88:721–726.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download