INTRODUCTION
Lower urinary tract symptoms (LUTS) and pelvic pain caused by diseases of the prostate have decreased the quality of life in men. Epidemiologic studies report that the prevalence of prostatitis-like symptoms is approximately similar to diabetes mellitus and ischemic heart disease. The rate of prostatitis-like symptoms was reported to range from 2.2% to 9.7%, and mean prevalence was 8.2% [1].
Prostatitis is a relatively common disease seen in the field of urology that reduces the quality of life of men in many ways. The National Institutes of Health (NIH) published that there are four categories in prostatitis, among which chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) corresponds to category III. According to the recommendation of the International Association for the Study of Pain, chronic pelvic pain syndrome and prostate pain syndrome have a similar definition and they are widely used recently [2]. A valid tool which can be used to assess CP/CPPS was developed as represented by the National Institutes of Health chronic prostatitis symptom index (NIH-CPSI) [3]. A previous study revealed that the prevalence of NIH-CPSI for the assessment of CP/CPPS is about 8%–10% [4].
Only 20 years ago, collecting prostatic secretion was performed to obtain bacterial culture, to confirm and treat bacterial causes of chronic prostatitis. Also, antibiotic treatment was prescribed for such symptoms [5].
In 1995, the Korea National Institute of Health announced clarification for prostatitis at the Diabetes and Digestive and Kidney Diseases (NIDDK) consensus meeting. However, in some studies, prescription of specific antibiotics failed to affect symptom improvement. As a 3rd classification category, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) presents voiding symptoms without urinary tract infection and chronic pelvic pain. In 1999, the chronic prostatitis symptom index(CPSI) announced the definition and classification supplemented CP/CPPS treatment manual based on previous treatment [6].From the beginning of the latter half of the 1900s and the early 2000s, some researchers reported that various treatment methods other than antibiotics are effective for treatment of CP/CPPS. Also, research was performed on randomized placebo- or sham-controlled treatment trials.
Benign prostatic hyperplasia and prostate cancer are typical prostate disease conditions and their diagnoses and treatment methods have significantly developed. Prostatitis are entities that need to be explored and have not been actively researched. Recently, some researchers proposed a new treatment method for prostatitis, particularly, CP/CPPS, and what type of method that was suggested will be observed.
The criteria for CP/CPPS could comprise several different types of symptoms. Shoskes et al. well outlined the UPOINT classification [7]. The categories are composed of urinary, psychosocial, organ specific, infection, neurologic, and skeletal muscle tenderness. Lately, the UPOINT system has been used to look at gathering of symptoms, to try to distinguish a common cause for some groups of patients. Two clusters have demonstrated the analysis of men with CP/CPPS using the UPOINT system, a ‘systemic group’ of infection, neurologic, and psychosocial and a ‘pelvic’ group with the domains of organ specific, urinary, and tenderness [8].
2. Cause of CP/CPPS
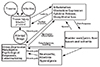
CP/CPPS affects quality of life adversely on the one hand and entails financial burden on the other. Various complex causes (urinary tract infection, prostatic urinary tract counterflow [9], Cytokines [10], pelvic floor spasm[11], systemic nervous or endocrine cause[12] and neuropsychiatric cause [13]) affects CP/CPPS in terms of pathological physiology (Fig. 1). Generally, these causes appear in a complex form, not a single one. Therefore, recently, breaking away from existing practice of monotherapy, the UPOINT phenotyping system that treats symptoms by discovering several causes of the condition in the patient has been introduced.
3. Management
3.1 Monotherapy
Several types of monotherapy have been introduced and researched for CP/CPPS. These treatment methods include alpha blockers, antibiotics, hormone therapy, anti-inflammatory treatment, plant therapy, anticonvulsant and non-pharmacological treatment. It was reported that these treatment methods are effective for treatment of CP/CPPS but there are other reports that such methods are not effective. In addition, research cases were limited and therapeutic methods that were available were not backed up by adequate research.
Alpha blockers have been researched as monotherapy and its therapeutic effect on CP/CPPS symptoms were reported in several theses. Nickel et al. reported in their research that Silodosin was effective for improvement of symptom and quality of life of in patients with CP/CPPS[14]. And it was further reported that terazosin[15] and alfuzosin[16] that are alpha blockers are also effective. However, according to a multicenter research performed at two places, their results presented the opposite. According to research done by Alexander et al., the NIH-CPSI score for 6-weeks of tamsulosin treatment did not show any improvement and in another large scaled research done on alfuzosin, it was reported that the advantage of alpha blocker was not represented and the symptom was not improved[17].
Antibiotics have been used for bacterial prostatitis almost essentially even in the case when culture tests were negative [18]. And there is also research reporting symptom improvement. In tetracycline research, symptom improvement was represented when comparing with the control group by using such an agent for 12 weeks. However, a survey on quality of life was not sufficient[19]. In a randomized controlled research, levofloxacin was used for 6 weeks and it was compared with placebo[20]. In total score of the NIH-CPSI, improved score between two groups was represented but its difference was not shown. In NIDDK-sponsored research, it was announced that ciprofloxacin was not superior to placebo[21]. In Meta-analysis for use of antibiotics[22], there was a research study that presented results that it may be clearly, individually effective, but not statistically and clinically. It is considered that CP/CPPS was caused by bacteria and that it may not be cultured in some patients or uncultured due to anti-inflammatory cytokine blocked antibiotics[23]. This result suggests that antibiotics treatment should not be recommended as first line therapy and this case is applicable particularly to a patient who fails antibiotic treatment previously.
Anti-inflammatory medicine has also been widely used for several symptoms of CP/CPPS and various drugs particularly including rofecoxib [24], pentosanpolysulfate [25], zafirlukast (a leukotriene antagonist) [26],prednisone [27], and tanezumab[28] have been researched. In case of administering Rofecoxib and celecoxib in high concentrations, symptom improvement was represented. Symptom improvement was evident in pentosanpolysulfate when its concentration was high but its CPSI score did not show clear improvement compared with control group. Zafirlukast and prednisone were not effective for symptom improvement and tanezumab also did not show any advantage. Anti-inflammatory treatment also did not show significant symptom improvement as monotherapy and it is not recommended as monotherapy as well. In plant treatment, quercetin was effective because antioxidant and material with anti-inflammatory function were contained in it[29]. In randomized, double-blind, placebo-controlled trials, the NIH-CPSI after quercetin treatment was lowered compared with control group and its side effect was also almost nil. Therefore, quercetin was somewhat effective as monotherapy.
In neuromodulatory treatment, pregabalin showed improvement in total score of NIH-CPSI and pain score but it was not significant statistically.
In hormone treatment, there is research using mepartricin that lowers estrogen levels[34] and research that uses finasteride[30]. Mepartricin research showed improvement in total NIH-CPSI score but its number was limited and the finasteride research showed symptom improvement compared with control group but it was not significant statistically. As such, hormone therapy was also not promising as monotherapy.
Regarding physical therapy, there is research showing results using Myofascial trigger point release. Research was performed on patients with CP/CPPS but the results were not significant statistically and its sample size was limited [31]. Each had merit/demerit as monotherapy and through active research, its efficacy was not demonstrated. Moreover, it was hard to treat patients with CP/CPPS by monotherapy and this treatment is unable to be applied clinically yet in the field where active research was not performed.
3.2 Development of UPOINT
Since a few research reporting that monotherapy was not effective were presented, a study was done on the efficacy of several treatments performed simultaneously, showing that it was more effective than monotherapy. Nickel et al. reported that when treating with alpha blocker, antibiotics or pain killing anti-inflammatory drug at the same time, the clinical score was more improved than control group and in other studies, it reported that when treating with alpha blocker, antibiotics and pain killing anti-inflammatory drug at the same time, it was more effective than monotherapy[32].
CP/CPPS was understood and developed by the NIH classification. Monotherapy was not effective for diversified syndromes of CP/CPPS. Therefore, it was insisted that the cause of several symptoms of CP/CPPS should be entirely clarified and that it be properly treated at the same time.
3.3 Treatment method in UPOINT perspective
It was learned that in order to properly and directly treat CP/CPPS, it is required to diagnose six kinds of clinical phenotype systems in the patients. Clinical six areas include urinary symptoms, psychosocial dysfunction, organspecific findings, infection, neurologic/systemic, and tenderness of muscles and it was named as UPOINT [7].
3.3-1 U: urinary
Silodosin had undergone randomized trials for treatment of CP/CPPS in 151 alpha-blocker-naive men. The CPSI score had shown dramatic change [36]. Other options such as tadalafil which is a PDE5 inhibitor, which can treat erectile dysfunction, lower urinary tract symptoms and possibly the symptoms of CP/CPPS as well [37].
3.3-2 P: psychosocial
Chung et al. studied the relationship between CP/CPPS and risk of subsequent depressive disorder in 18,306 patients with a prospective, population-based study over a 3-year follow-up. CP/CPPS was a significant precursor for progression of depressive disorder with a hazard ratio of 1.63 [95% confidence interval (CI) 1.36–1.96] [38]. Signs of depressive disorder and catastrophizing (helplessness and hopelessness about the condition) can excite conveying psychological assessment. Some researchers are developing cognitive-based therapy to express these issues in addition to traditional therapy [39].
These types of programs provide a positive method of treatment for the future, although not yet widely available.
3.3-3 O: organ-specific
The REDUCE study used 0.5mg dutasteride versus placebo in men at risk (age 50–75, prostate specific antigen 2.5–10 ng/ml with negative transrectal ultrasound). A 4-year, randomized, double-blind, placebo-controlled study of prostate cancer risk reduction was performed. Finasteride and dutasteride which are hormonal agents regarding symptoms of CP/CPPS involve improved voiding symptoms, regression of glandular tissue, and minimized intraprostatic ductal reflux, or any one of them. Radiofrequency hyperthermia is also organ-specific treatment. One-hundred five patients, treated with transrectal radiofrequency hyperthermia with or without antibiotics for 6 weeks had a considerable improvement in the domains of the NIH-CPSI compared to the patients using antibiotics alone [40].
3.3-4 I: infection
Symptoms of CP/CPPS overlap with those of prostatic infection even if in the definition of CP/CPPS it specifically states that it must be in the absence of an uropathogenic source. Four weeks therapy of antibiotics is an accepted treatment [41]. In the absence of a positive urine culture, giving repeated antibiotics is not accepted therapy. A study from Washington University in St. Louis raised the question of whether treating infection in the gut would also help symptoms of CP/CPPS [42]. Patients with CP/CPPS were evaluated using the lactulose breath test (LBT) for small intestinal bacterial overgrowth (SIBO). Rifaximin was used to on patients with positive LBT (14 of 16 patients screened), a gut-directed antibiotic, for 10 days. At 18 days after the end of treatment, mean NIH-CPSI score diminished significantly from 25 to 21. Abdominal pain and bloating also decreased.
3.3-5 N: neurologic/systemic
Study of pregabalin, an anticonvulsant that is approved for neuropathic pain such as postherpetic neuralgia, diabetic neuropathy, and fibromyalgia, had an effect that approached significance, but did not make the primary endpoint [43]. However, secondary endpoints were significantly improved. This means that pregabalin may be effective in a subset of men with CP/CPPS. A placebo-controlled study, Tenazumab, a monoclonal antibody against nerve growth factor, in men with CP/CPPS has been reported recently [44]. At week 6, no difference in overall NIH-CPSI score with marginal advancement in average daily pain and urgency frequency was found when compared to placebo.
3.3-6 T: tenderness of skeletal muscle
Pelvic floor dysfunction area has seen the greatest progression in diagnosis and treatment. Tight muscles in the pelvic floor lead to pain. The group from Stanford has made significant contributions in pelvic floor dysfunction on myofascial release and treatment [45]. Pelvic floor physical therapy techniques are an important part of the treatment of many men with CP/CPPS, thus, the physical therapist plays an important role.
Evaluation is performed by each area using the NIH-CPSI. The NIH-CPSI shows total sum by indicating each area and it has relations with the duration of symptoms. It is considered that UPOINT would provide significant useful contributions to multimodal therapy using clinical phenotypes.
Recently, in Germany and Canada, they observed that when evaluating UPOINT by including sexual dysfunction, the NIH-CPSI evaluation was performed more in detail [46]. It was insisted that CP/CPPS may also affect erectile dysfunction. However, it was reported that evaluating sexual dysfunction by including it in UPOINT is not significant [47].
Clinical research on UPOINT was limited but recently in a prospective study, when treating simultaneously after evaluating by each item, NIH-CPSI score after 6 months was decreased by at least 6 points in 84% patients and its average score was decreased by 12 points. [48].
UPOINT therapy is an attractive treatment method for the patients who were diagnosed as CP/CPPS and suffering from pain. Each treatment item and method were summarized in Table 1. Treatment shall be performed after identifying the applicable item. For example, in the case where a patient has urination symptoms and organ pain, it should be treated with alpha blocker and quercetin and if other neurological pain accompanied, pregabalin and pelvis physical therapy prescription are required.
For the past 15 years, treatment method for CP/CPPS has been extensively researched but its clinical treatment was disappointing to both patients and doctors. Specific monotherapy for CP/CPPS is not effective and not recommended. Therefore, randomized placebo- and sham-controlled trials are required for more extensive research to discover which treatment method is more effective individually. And it would be more effective to perform concurrent treatment by clarifying clinical phenotype symptom of the patient in detail [49].
3.4 Limitation of UPOINT
Approach to UPOINT was encouraged by research results showing that medicine (alpha blockers, anti-inflammatory drug) being used as existing monotherapy was not effective for CP/CPPS treatment. However, clinical research on UPOINT was limited and its mechanistic treatment principle was not clarified. Moreover, a study on or inter-domain showing symptoms by patient was not performed. Furthermore, because long-term evaluation for the treatment was not performed, there are many tasks to be done in further research and its efficacy should be also verified [50].
4. Conclusion
Physicians can give multimodal therapy for patients with CP/CPPS according to its clinical phenotype, and several clinical studies have demonstrated obvious clinical benefit from the UPOINT-based therapy. Pain related to CP/CPPS includes diversity factors that influence the experience. There are also associated pain syndromes in some patients who must be diagnosed and addressed. The best treatment is considering different aspects of the condition.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download