Abstract
Objective tinnitus originates from the para-auditory structures of the head and neck and can be heard or documented by examiner. Three representative forms of objective tinnitus, according to the causal organs are myoclonic tinnitus, vascular tinnitus and tinnitus caused by the patulous Eustachian tube. Etiologies, pathologic mechanisms, diagnostic approaches, and proper treatment methods of objective tinnitus are comprehensively discussed with a review of literatures. Objective tinnitus can be cured in many cases. Clinicians need to be well aware of the clinical characteristics of objective tinnitus since early, correct diagnosis with proper management are mandatory for its cure.
Objective tinnitus or somatosound originates from the para-auditory structures of the head and neck. This symptom is much less common compared to subjective tinnitus or sensorineural tinnitus, and the sound or etiologies can be heard or observed by both the patient and examiner. Conditions that can cause objective tinnitus include tinnitus of muscle origin, vascular origin and a patulous eustachian tube. A previous report investigating the incidence of objective tinnitus has demonstrated over 17% among 211 tinnitus patients who visited a tinnitus clinic in a tertiary referral center [1]. Once properly diagnosed, objective tinnitus can be more attractive to the clinician due to its curability. Therefore, etiologic factors should be thoroughly evaluated to achieve the best therapeutic results in the management of objective tinnitus. Careful history taking, mindful physical examination to identify the possible causes, audiologic, medical, and radiological evaluations for each type of objective tinnitus are needed for the proper diagnosis and treatment.
Myocloinic tinnitus can be subdivided to palatal myoclonic tinnitus and middle ear myoclonic tinnitus. Based on the muscle volume and location, palatal myoclonic tinnitus is expressed as a mostly irregular, non-continuous clicking sound, whereas middle ear myoclonic tinnitus is characterized as a rather soft crackling or buzzing sound.
Palatal myoclonic tinnitus is an extremely rare type of tinnitus caused by voluntary or involuntary contraction of palatal muscle. Two discrete forms have been identified, namely a 'symptomatic'form or an 'essential' form. A symptomatic palatal myoclonic tinnitus which develops secondary to tumorous, ischemic or degenerative lesions of the brainstem or the cerebellum is less common and is usually observed in middle aged patients with a predominant tendency to males [2]. Essential palatal myoclonic tinnitus in which no structural lesion is discernable, is more common and prominent than the secondary form, and usually presents to otolaryngologists with the symptom of a loud clicking type of tinnitus. It commonly occurs with a mean age of 20s but may also occur in childhood with the same gender proportion and mostly continues without therapy; however, may disappear spontaneously in a rare occasion. The etiology of either form of palatal myoclonus still remains unclear and presumably involves a dysfunction (essential) or a lesion (symptomatic) of the connections between the dentate nucleus, red nucleus and inferior olivary nuclei, which is called the Guillain-Mollaret triangle.
The proper diagnosis of essential or secondary palatal myoclonic tinnitus is most often made by the observation of palatal myoclonic movement under endoscopic examination and the auscultation of clicking tinnitus with Toynbee tube. Neurological examination and imaging study are necessary to exclude a brain lesion which would lead to a diagnosis of secondary palatal myoclonus. Hyperactive palatal myoclonic contraction can be detected and documented by an electromyogram (EMG) (Fig. 1A). Several therapeutic strategies have been demonstrated to relieve symptomatic clicking tinnitus with varying therapeutic results of success and/or adverse effects.
Medical treatment has used anti-convulsatants, muscle relaxants, benzodiazepines, barbiturates, and anti-cholinergic agents, but no drug has been shown to significantly improve symptoms reproducibly. Surgical therapies including sections of the levator veli palatini muscle and the tensor veli palatini muscle, have also provided highly variable results and also run a high risk of side effects. Recently, the most popular, safe and effective therapeutic tool for palatal myoclonic tinnitus has been the injection of botulinum toxin to the palatal muscles. Promising results have been shown in various case reports (Table 1) [3456]. Known side effects of botulinum toxin injection to palatal muscles are velopharyngeal insufficiency, hypernasal voice (rhinophonia), and temporary dysphagia due to the excessive neuromuscular inhibition of the adjacent muscles of the palate. To reduce side effects, toxin dosage should be as high as necessary, but as low as possible. EMG-guided injection can be useful to achieve effective injection of toxin with the avoidance of side effects to target the muscles of the palatal myoclonic tinnitus (Fig. 1B). Recent data from our tinnitus clinic has shown the development of essential palatal myoclonic tinnitus in younger ages (mean 27.9 years) without gender difference. Many of these cases were accompanied by middle ear myoclonic tinnitus (43.9%). Conservative therapy, using behavioral therapy and reassurance along with medication using anticonvulsants, muscle relaxants, and anxiolytics for 3 months has demonstrated satisfactory results in over 80% of the patients. However, patients with intractable palatal myoclonic tinnitus were treated by botulinum toxin injection, either EMG-guided or not, and were completely cured without significant long lasting side effects [7].
Tinnitus secondary to middle ear myoclonus is very rare, and consequently there are very few reports of it in the English literature [89101112]. It has been suggested that the crackling or buzzing sounds reported are caused by repetitive and abnormal tensor tympani and stapedial muscles contractions [11]. These myoclonic movements are thought to be a form of segmental myoclonus involving brainstem innervated muscles. Vascular, infectious and demyelinating disorders as well as anxiety, trauma and neoplastic disease have all been implicated in the etiology of segmental myoclonus [91112]. In a previous report of the largest case series of middle ear myoclonus, 58 enrolled patients had a mean age of 29.8 years (range, 6-70 years) and 20.7% (n=12) were less than 10 years old [12]. Stressful events or noise exposure were frequently associated with the onset of middle ear myoclonic tinnitus, which indicates that a possible pathological mechanism of the middle ear myoclonic tinnitus could be brain reorganization due to stress, noise or brain lesions. Middle ear myoclonic tinnitus can also be associated with vigorous contractions of the eyelid, called forceful eyelid closure syndrome [14], or with palatal myoclonus [12].
Impedance audiogram and otoendoscopic examinations of the tympanic membrane are helpful tools for diagnosing middle ear myoclonic tinnitus. Inward-and-outward motion, mostly in the posterior part of the tympanic membrane can be observed on otoendoscopic examination. Sound evoked tremor-like motion of the tympanic membrane, which is also frequently observed in an acoustic reflex decay test or strong inward-and-outward motion of the tympanic membrane in the middle ear myoclonic tinnitus associated with forceful eyelid closure syndrome are useful objective tools for diagnosing MEM tinnitus (Fig. 2). Tympanometry can confirm rhythmic changes in the middle-ear compliance in patients with middle ear myoclonic tinnitus [13]. Diagnostic tools useful for middle ear myoclonic tinnitus are summarized in Table 2.
Medical treatment with an anticonvulsant, i.e. cabarmazepine, a muscle relaxant or a sedative agent have all been reported to produce partial to complete relief of symptoms in middle ear myoclonic tinnitus [1415].
In addition, more than 75% of patients exhibited complete or partial remission of their symptom using combination medical therapy [12]. Other therapeutic options like white band noise masking devices were also successful in treating middle ear myoclonus [16]. Botulinum toxin placed on a piece of gelfoam socked form and inserted through a perforation of the tympanic membrane into the middle ear cavity of a patient suffering stapeius myoclonic tinnitus was able to provide disappearance of symptoms for 3 months, indicating the possible therapeutic tool of botox toxin for middle ear myoclonic tinnitus [17]. Patients with intractable middle ear myoclonic tinnitus who underwent sectioning of the middle ear tendons showed very good outcomes [18]. Sectioning of middle ear tendons (both tensor tympani and stapedius) via a transmeatal approach in 11 ears with intractable tinnitus resulted in complete resolution or marked improvement of the symptom in all cases, demonstrating surgical sectioning of middle ear tendons to be successful in treating intractable middle ear myoclonic tinnitus [12]. Considering the high response rate to medical therapy, clinicians should prescribe medication as a first-line management of this disorder. If medical therapy fails, surgical sectioning of the tendons of the middle ear muscles is a good second-line management procedure.
Vascular tinnitus is the most common form of pulsatile tinnitus, particularly when the tinnitus corresponds with the pulse of patients. It is an uncommon otologic symptom; the incidence has been reported to be approximately 4% in patients with tinnitus [19]. Vascular tinnitus originates from vascular etiologies and may occur as a result of turbulence in the blood flow and can be classified as either arterial or venous type [20]. The flow of blood in a smooth-walled blood vessel is laminar and is usually silent. Noise may result when laminar flow in the blood vessel is disturbed and the resultant turbulence is thought to cause a vibration of the vessel walls that finally is detected as pulsatile tinnitus by patients. It is sometimes heard in normal adults with hypertension or hyperdynamic circulatory states [21].
Etiologies of vascular tinnitus or pulsatile tinnitus are listed in Table 3. The incidence of vascular tinnitus reported in a relatively large case series demonstrated that the most common cause was a high jugular bulb, and the second most common was venous hum. Arterial causes were less common. Glomus tympanicum, intracranial hemangioma, arteriovenous malformation and aberrant carotid artery, and benign intracranial hypertension (BIH) were other etiologic factors of vascular tinnitus in this study [22]. In another previous study, BIH was the most common diagnosis (56 patients) of vascular tinnitus [23]. It has been reported that the incidence of BIH is increased in women who are 10% or more over ideal weight, and varies according to the prevalence of obesity in the respective region [24].
A detailed history of pulsatile tinnitus is essential to diagnose the possible cause of vascular tinnitus. Throbbing, rushing, or humming sounds synchronized with the pulse suggests a vascular etiology. Thorough physical examinations through otoscopy, auscultation of the ear canals, and a full head and neck examination should be performed. A tympanic mass could be visible on otoscopy or microscopic examination. To discriminate venous causes of vascular tinnitus, the ipsilateral internal jugular vein (IJV) can be gently compressed and any changes in tinnitus loudness reported. A head turning test is also used to confirm the venous etiologic vascular tinnitus along with the compression maneuver. In tinnitus of venous origin, light digital pressure over the ipsilateral IJV or head turning towards the tinnitus side elicits the tinnitus to diminish or subside.
Audiometric tests should include pure tone audiometry and tympanometry. Tympanometry is useful to diagnose middle ear effusion and other associated conductive hearing loss. Auditory evoked brainstem responses are useful in patients suspected of having benign intracranial hypertension in which cases prolonged interpeak latencies are the most common abnormal findings [25]. A full blood count, vitamin B12, and thyroid function tests should be performed to exclude hyperhemodynamic causes of anemia or hyperthyroidism. Cholesterol level is useful to check for hyperlipidemia, a risk factor for atherosclerosis.
To select an imaging study, the potential etiology of venous or arterial origin can be a determining factor for choice of imaging. Temporal bone computed tomography, or brain magnetic resonance venography (MRV) would be a first line of imaging study for venous-origin vascular tinnitus whereas transcranial Doppler sonography or brain CT angiography or brain magnetic resonance angiography (MRA) will differentiate arterial-origin vascular tinnitus (Fig. 3) [26]. For the rare cause of vascular tinnitus, an aneurysm of brain vessels, angiographic confirmation followed by concomitant radiological intervention with coil or other materials have been reported [27].
A diagnostic algorithm for vascular tinnitus has been suggested in our previous report [22]. Pulsatile tinnitus should be called idiopathic only after an extensive work-up has been completed and a specific diagnosis has not been reached. Thorough history taking and physical examination as well as individualized diagnostic testing are important factors in evaluating patients with pulsatile tinnitus (Table 4).
Treatment for vascular tinnitus should be individualized according to the etiologic factors.
Treatment methods for high jugular bulb-origin vascular tinnitus could include pharmacological intervention to reduce hyperhemodynamic conditions, or anxiety relieving treatments as well as tinnitus retraining therapy, sound therapy and cognitive behavioral therapy [23]. In cases of the failure of initial treatment, surgical therapy such as jugular vein ligation, transcatheter endovascular coil embolization, transvenous stent-assisted coil embolization and surgical covering and reinforcement using fascia, perichondrium, or autologous cartilage can be considered although they are not always successful [28]. Moreover, potential complications of surgical management such as intracranial hypertension, low cranial nerve palsy or conductive hearing loss should be considered.
Venous hum can be treated by conservative medical therapy with antidepressant and/or anxiolytics first, as well as tinnitus retraining therapy; other surgical therapy has not been shown as effective. Benign intracranial hypertension can be managed by reducing body weight and administration of acetazolamide and furosemide [29]. Pulsatile tinnitus in patients with hypertension should be managed by controlling their hypertension with proper medication. Treatment of patients with glomus tumors is mainly surgical excision. Carotid endarterectomy for patients with atherosclerotic carotid artery disease should be considered when obstruction is more than 60% [30]. Selective embolization techniques for arterio-venous fistulae or intracranial aneurysm showed the excellent therapeutic results of not only life-saving but also of symptomatic management of pulsatile tinnitus. Vascular tinnitus could be alleviated or cured in most of the patients with the individualized treatment according to the etiologic factors. Thorough evaluation and proper diagnosis are mandatory for relieving or curing pulsatile tinnitus in patients with vascular tinnitus.
Patulous Eustachian Tube (PET) was first described by Schwartze in 1864 and can result in multiple symptoms of autophony of voice and breathing and aural fullness [31]. It is an abnormal patency of the eustachian tube that affects 0.3% to 6.6% of people [32]. Since most of the patients with PET report symptoms of perception of one's own voice or physiologic sound due to the patulous condition of the Eustachian tube (ET), PET has been classified as a type of objective tinnitus.
Several etiologies of PET have been proposed, including loss of tissue within the cartilaginous portion of ET caused by weight loss, pregnancy, use of high-dose oral contraceptives and estrogen therapy. Atrophy or scarring of the Eustachian tube or nasopharynx caused by adenoidectomy, radiation therapy or other iatrogenic trauma also can be associated with altered eustachian tube function [3334].
The diagnosis of PET is made clinically on the basis of subjective characteristic symptoms and signs. Criteria of PET include autophony of voice and respiration as well as aural fullness. Due to the symptom of autophony, many patients with PET complain of difficulty in everyday conversation which often causes severe stress and even depression. This type of objective tinnitus can be diagnosed by physical examination and audiometric test. Tympanic membrane movement on respiration and severe perturbation of the tympanogram checked during respiration have been used as good confirmatory diagnostic methods (Fig. 4).
Various therapeutic attempts for PET have been reported with various success rates and results (Table 5).
Saline was the most commonly used topical treatment, but historical formulas such as salicylic and boric acid powder (in a 1:4 proportion), diluted hydrochloric acid, chlorobutanol, and benzyl alcohol and saturated potassium iodide solution have been studied [353637].
Results ranged from 63.5% to 100% partial or full resolution of symptoms without consistent effectiveness among the previous reports.
The symptoms of PET are postulated to arise from tympanic membrane movement, thus techniques to weigh down the TM have been attempted. Tympanostomy tube insertion was the most frequently described method with the results ranging from 53-100% of patients achieving partial or full resolution of the symptoms of PET [3538]. Sniffing to alleviate PET symptoms should be requested and the attic retraction of the tympanic membrane as well as the inward-and-outward motion of it should be checked to detect the relationship between sniffing and PET. Ikeda et al. reported the tympanostomy tube insertion was more effective in patients of PET with sniffing habit than those without [35]. No serious adverse events except temporary otorrhea or acute otitis media have been reported.
Recently, mass loading of the tympanic membrane using tympanic membrane paper patching has been reported with the success rate of PET symptom control up to 76.2% [39]. Patients responding to the paper patching of tympanic membrane can be treated with permanent augmentation of the tympanic membrane with cartilage tympanoplasty to alleviate PET symptoms [40]. Patients who exhibit a flaccid tympanic membrane segment with the symptom of PET and are good responders to paper patching are more appropriate for cartilage tympanoplasty.
To prevent the movement of air through an abnormally open ET, transtympanic, transnasal or transoral occlusion of the ET opening has been attempted. Different techniques such as ET plugging, cautery of the ET orifice, or injection of bulking substance at the ET orifice have been introduced with variable therapeutic results [41424344]. Plugging of the ET via transtympanic or transnasal approaches has been reported and the devices used include transvenous angiocatheter or silicone plug [424344]. Eustachian tube injection was frequently applied to PET patients as a method of occlusion of ET orifice. Injectable materials included Teflon, silicone, gelatin sponge, a polytef paste, fat and cartilage [454647]. Cautery of the ET orifice to promote scarring using silver nitrate, diathermy or KTP laser has been introduced to relieve PET symptoms with mixed results. Serous otitis media were reported as a complication with the ET cautery procedure [484950].
To date, there has been no simple and single satisfactory solution for patients with PET. However, with the proper diagnosis of PET, patients suffering from their aural fullness and autophony can be managed successfully with various types of therapeutic modalities.
Objective tinnitus can be a very distressing, but mostly curable symptom. Clinical history and physical examination are most important in establishing the correct diagnosis. Audiometric, laboratory and imaging studies appropriately selected for the patient can aid or confirm diagnosis. Treatment of objective tinnitus should be based on a comprehensive diagnosis of the etiologic and concomitant aspects of an individual's tinnitus. A large variety of therapeutic interventions are available for each type of objective tinnitus, which can efficiently reduce tinnitus severity or even cure the tinnitus. To reach the best therapeutic result of objective tinnitus, early, proper diagnostic approaches and selection of the most efficient therapeutic modality should be applied to each patient as a customized method.
Figures and Tables
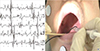 | Fig. 1The pre-injection electromyogram of the the tensor veli palatini muscle showed the bursts of electrical activity with different frequencies and amplitudes (A). Botulinum toxin injection to the tensor veli palatini muscle was accomplished through an EMG-guided external approach (B). |
 | Fig. 2Diagnostic methods of middle ear myoclonic tinnitus. Direct observation of To-and-fro, or inward-and-outward motion, mostly in the posterior part of the tympanic membrane by otoendoscopic examination (A, arrows), sound evoked tremor-like motion of tympanic membrane which is also frequently observed in acoustic reflex decay test (B) are useful tools. Strong inward-and-outward motion of tympanic membrane in middle ear myoclonic tinnitus associated with a forceful eyelid closure syndrome can be documented even in a static compliance test (C). |
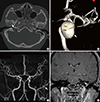 | Fig. 3Vascular tinnitus diagnosed by imaging studies (arrows). High jugular bulb detected in temporal bone computed tomogram (A), intracranial aneurysm in 3D-reconstructed Brain CT angiogram (B), arteriovenous fistula in magnetic resonance arteriography (C), and glomus tympanicum in Gd-enhanced temporal bone magnetic resonance imaging (D). |
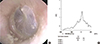 | Fig. 4Diagnosis of the patulous Eustachian tube. Otoendoscopic examination of the tympanic membrane during forceful inspiration and expiration with the contralateral nostril blocked demonstrates the inward-and–outward motion of the total tympanic membrane according to the respiratory status. Outward buldging of tympanic membrane during expiration is shown in this otoendoscopic finding (A). It can also be documented by tympanogram in accordance with forceful respiration which shows severe and irregular perturbation (B). |
References
1. Yoo HJ, Park SN, Kim DK, Park KH, Kim MJ, Kim JE, et al. Incidence and clinical characteristics of patients with tinnitus according to diagnostic classification. Korean J Otorhinolaryngol-Head Neck Surg. 2011; 54:392–398.

2. Duschel G, Mischeke G, Schenk E, Shulte-Monting J, Luking CH. Symptomatic and essential rhythmic palatal myoclonus. Brain. 1990; 113:1645–1672.

3. Saeed SR, Brookes GB. The use of clostridium botulinum toxin in palatal myoclonus. A preliminary report. J Laryngol Otol. 1993; 107:208–210.

4. Penney SE, Bruce IA, Saeed SR. Botulinum toxin is effective and safe for palatal tremor: a report of five cases and a review of the literature. J Neurol. 2006; 253:857–860.

5. Park SN, Park KH, Kim do H, Yeo SW. A case of palatal myoclonus associated with orofacial buccal dystonia. Clin Exp Otorhinolaryngol. 2012; 5:44–48.

6. Park SN, Park KH, Lee DH, Yeo SW. A Case of Palatal Myoclonus Tinnitus Treated with Botolinum Toxin Injection . Korean J Otolaryngol-Head Neck Surg. 2005; 48:1177–1180.
7. Park SN. Treatment f objective tinnitus. In : Kyung Hee Tinnitus Seminar 7th; Tinnitus treatment in special situation;2015.
8. Golz A, Fradis M, Martzu D, Netzer A, Joachims HZ. Stapedius muscle myoclonus. Ann Otol Rhinol Laryngol. 2003; 112:522–524.

9. Marchiando A, Per-Lee JH, Jackson RT. Tinnitus due to idiopathic stapedial muscle spasm. Ear Nose Throat J. 1983; 62:8–13.
10. Park SN, Nam IC, Shin JH, Yeo SW. A case of objective tinnitus due to middle ear myoclonus treated by surgical therapy. Korean J Otolaryngol-Head Neck Surg. 2007; 50:73–75.
11. Lee GH, Bae SC, Jin SK, Park KH, Yeo SW, Park SN. Middle ear myoclonus associated with forced eyelid closure in children: diagnosis and treatment outcome. Laryngoscope. 2012; 122:2071–2075.

12. Park SN, Bae SC, Lee GH, Song JN, Park KH, Jeon EJ, et al. Clinical characteristics and therapeutic response of objective tinnitus due to middle ear myoclonus: a large case series. Laryngoscope. 2013; 123:2516–2520.

13. Abdul-Baqi KJ. Objective high-frequency tinnitus of middle-ear myoclonus. J Laryngol Otol. 2004; 118:231–233.

14. Badia L, Parikh A, Brookes J. Management of middle-ear myoclonus. J Laryngol Otol. 1994; 108:380–382.
15. Abdul-Baui KJ. Objective high-frequency tinnitus of middle-ear myoclonus. J Laryngol Otol. 2004; 118:231–233.

16. East CA, Hazel JW. The suppression of palatal (or intra-tympanic) myoclonus by tinnitus masking devices. J laryngeal Otol. 1987; 101:1230–1234.

17. Liu HB, Fan JP, Lin SZ, Zhao SW, Lin Z. Botox transient treatment of tinnitus due to stapedius myoclonus: case report. Clin Nuerol Neurosurg. 2011; 113:57–58.

18. Zipfel TE, Kaza SR, Green JS. Middle ear myoclonus. J Laryngol Otol. 2000; 114:207–209.
19. Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Disord. 1990; 55:439–453.

20. Liyanage SH, Singh A, Savundra P, Kalan A. Pulsatile tinnitus. J Laryngol Otol. 2006; 2. 120(2):93–97.

21. Hardison JE, Smith RB, Crawley IS. Self heard venous hums. JAMA. 1981; 245:1146–1147.
22. Bae SC, Kim DK, Yeo SW, Park SY, Park SN. Single-center 10-year experience in treating patients with vascular tinnitus: diagnostic approaches and treatment outcomes. Clin Exp Otorhinolaryngol. 2015; 8:7–12.

23. Sismanis A. Pulsatile tinnitus: a 15-year experience. Am J Otol. 1998; 19:472–477.
24. Radhakrishnan K, Thacker AK, Bohlaga NH, Maloo JC, Gerryo SE. Epidemiology of idiopathic intracranial hypertension: a prospective and case-control study. J Neurol Sci. 1993; 5. 116:18–28.

25. Reid A, Marchbacks RJ, Bateman DE, Martin AM, Brihgtwell AP, Pickard JD. Mean intracranial pressure monitoring by a non-invasive audiological technique: a pilot study. J Neurol Neurosurg Psychiatry. 1989; 52:610–612.

26. Kim DK, Shin YS, Lee JH, Park SN. Pulsatile tinnitus as the sole manifestation of an internal carotid artery aneurysm successfully treated by coil embolization. Clin Exp Otorhinolaryngol. 2012; 5:170–172.

27. Austin JR, Maceri DR. Anterior communicating artery aneurysm presenting as pulsatile tinnitus. ORL. 1993; 55:54–57.

28. Park SN, Park KH, Lim DJ, Lee JH. A case of pulsatile tinnitus with high jugular bulb treated by ligation of internal jugular vein. Korean J Otorhinolaryngol-Head Neck Surg. 2007; 50:1152–1156.
29. Sesmanis A. Otologic manifestations of benign intracranial hypertension syndrome: diagnosis and management. Laryngoscope. 1987; 97:1–17.

30. Executive committee for the asymptomatic carotid atherosclerosis study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 273:1421–1428.
31. O'Connor AF, Shea JJ. Autophony and the patulous eustachian tube. Laryngoscope. 1981; 91:1427–1435.
32. DiBartolomeo JR, Henry DF. A new medication to control patulous eustachian tube disorders. Am J Otol. 1992; 13:323–327.
33. Plate S, Johnsen NJ, Nodskov Pedersen S, Thomsen KA. The frequency of patulous eustachian tubes in pregnancy. Clin Otolaryngol Allied Sci. 1979; 4:393–400.

34. Pulec JL. Abnormally patent eustachian tubes: treatment with injection of polytetrafluoroethylene (teflon) paste. Laryngoscope. 1967; 77:1543–1554.

35. Ikeda R, Oshima T, Oshima H, Miyazaki M, Kikuchi T, Kawase T, et al. Management of patulous eustachian tube with habitual sniffing. Otol Neurotol. 2011; 32:790–793.

36. Oshima T, Kikuchi T, Kawase T, Kobayashi T. Nasal instillation of physiological saline for patulous eustachian tube. Acta Otolaryngol. 2010; 130:550–553.

37. Boudewyns A, Claes J. Acute cochleovestibular toxicity due to topical application of potassium iodide. Eur Arch Otorhinolaryngol. 2001; 258:109–111.

38. Chen DA, Luxford WM. Myringotomy and tube for relief of patulous eustachian tube symptoms. Am J Otol. 1990; 11:272–273.
39. Boedts M. Paper patching of the tympanic membrane as a symptomatic treatment for patulous eustachian tube syndrome. J Laryngol Otol. 2014; 128:228–235.

40. Brace MD, Horwich P, Kirkpatrick D, Bance M. Tympanic membrane manipulation to treat symptoms of patulous Eustachian tube. Otol Neurotol. 2014; 35:1201–1206.

41. Boudewyns A, Claes J. Acute cochleovestibular toxicity due to topical application of potassium iodide. Eur Arch Otorhinolaryngol. 2001; 258:109–111.

42. Yu BJ, Kim HM, Jin SK, Park SN. Therapeutic efficacy of angiocatheter insertion surgery in the bony orifice of patulous Eustachian tube. Korean J Otorhinolaryngol-Head Neck Surg. 2014; 57:54–60.

43. Bluestone CD, Cantekin EI. "How I do it"-otology and neurotology. A specific issue and its solution. management of the patulous eustachian tube. Laryngoscope. 1981; 91:149–152.
44. Sato T, Kawase T, Yano H, Suetake M, Kobayashi T. Trans-tympanic silicone plug insertion for chronic patulous eustachian tube. Acta Otolaryngol. 2005; 125:1158–1163.

45. Takano A, Takahashi H, Hatachi K, Yoshida H, Kaieda S, Adachi T, et al. Ligation of eustachian tube for intractable patulous eustachian tube: a preliminary report. Eur Arch Otorhinolaryngol. 2007; 264:353–357.

46. Doherty JK, Slattery WH 3rd. Autologous fat grafting for the refractory patulous eustachian tube. Otolaryngol Head Neck Surg. 2003; 128:88–91.

47. Oh SJ, Lee IW, Goh EK, Kong SK. Endoscopic autologous cartilage injection for the patulous eustachian tube. Am J Otolaryngol. 2016; 37:78–82.

48. O'Connor AF, Shea JJ. Autophony and the patulous eustachian tube. Laryngoscope. 1981; 91:1427–1435.
49. Robinson PJ, Hazell JW. Patulous eustachian tube syndrome: the relationship with sensorineural hearing loss. J Laryngol Otol. 1989; 103:739–742.

50. Yanez C, Pirron JA, Mora N. Curvature inversion technique: a novel tuboplastic technique for patulous eustachian tube—a preliminary report. Otolaryngol Head Neck Surg. 2011; 145:446–451.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download