Abstract
Various microrobots are being studied for potential biomedical applications including targeted cell transportation, precise drug delivery, opening blocked blood vessels, micro-surgery, sensing, and scaffolding. Precise magnetic field control system is a coil system for wireless control of those microrobots for personalized and minimally invasive treatments. The microrobots for possible biomedical applications are fabricated by micro-electro-mechanical systems (MEMS) and nano-electro-mechanical systems (NEMS) technologies. In this review, fabrication technologies for scaffold and ciliary microrobots will be introduced and their control methods will be discussed. Various materials are being used for the fabrication of the microrobot such as SU-8, IP-Dip, IP-L, silicon, etc. The scaffold and ciliary microrobots are fabricated by SU-8, IP-Dip, and IP-L because these materials showed the maximum performance for three-dimensional (3D) microrobots using a 3D laser lithography system. All or part of the structures are coated with nickel and titanium layers after fabrication of the structures for magnetic control and biocompatibility, respectively, of the microrobots.
A microrobot is a tiny structure that can be remotely controlled to perform a mission which is a definition of a robot. In general, the size of a microrobot is from few micrometers to few millimeters. Microrobots for biomedical applications should be able to swim in a fluidic environment of the body including circulatory, the urinary, and nerve system by external magnetic fields and field gradient [1234]. These microrobots are expected to perform various biomedical applications including targeted cell transportation, precise drug delivery, opening blocked blood vessels, micro-surgery, sensing, and scaffolding [123456]. Among these applications, targeted drug delivery and cell transportation can be implemented by fabrication of biocompatible and magnetically controllable microrobots [236]. The structures of the microrobots can be fabricated by various materials such as SU-8, IP-Dip, IP-L, silicon, etc. [7891011] Especially, SU-8, IP-Dip, and IP-L are being used to fabricate precise three-dimensional (3D) microrobots using a 3D laser lithography system [248911121314]. The polymer structures of the microrobots should be coated with nickel and titanium layers after fabrication for magnetic wireless control and biocompatibility of the microrobots [2911]. The microrobots with a magnetic layer can be precisely controlled by external magnetic fields generated by a magnetic coil system. Relevant magnetic fields or field gradient should be used to control a microrobot based on the driving mechanism of each microrobot [15161718]. Many research works have been focused on only implementation of locomotion with a simple structure or a magnet because of the difficulty of fabrication and assembly of small structures. In this review, 3D laser lithography will be briefly introduced to explain the fabrication method for some of the biomedical microrobots. The driving mechanism for each microrobot will also be introduced with two magnetic control systems. Finally, the results of the precise control of the microrobots will be presented and show the possibilities of the microrobot for biomedical applications.
Micro-electro-mechanical systems (MEMS) and nano-electro-mechanical systems (NEMS) technologies were adopted to fabricate the structures of various microrobots. One of the new approaches for the fabrication of 3D porous microrobots is a 3D laser lithography using photo-curable polymers such as SU-8, IP-Dip, and IP-L. SU-8 is a negative photoresist for two-dimensional (2D) lithography to transfer patterns on a photo-mask by selective exposure of ultraviolet (UV) light. IP-Dip and IP-L are special tailored photoresists with maximum performance for the 3D laser lithography system [192021]. The feature created using SU-8 is relatively bigger than the size of the designed feature size [22]. Compared to SU-8, IP-Dip and IP-L provide the highest resolution with the minimum feature size down to 150 nm [22].
The fabrication preprocess for the 3D laser lithography system is shown in Fig. 1 (Photonic professional, Nanoscribe GmbH, Germany). First, the glass wafer is cleaned and dried to be used as a substrate and a drop of negative photoresist (PR) was released on the substrate as depicted in Fig. 1(a, b). Writing a 3D structure on the negative PR is explained in Fig. 1(c) and development was performed to remove the unexposed part of the PR as shown in Fig. 1(d). Finally, 170 nm nickel and 20 nm titanium layers were deposited by a sputter (SORONA Co. Ltd., Korea) for magnetic control and biocompatibility of the microrobot, respectively. Fig. 1(f) shows the fabricated structures after laser parameter test to find the optimized parameters for laser intensity and writing speed.
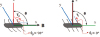
The fabricated microrobots coated with nickel have magnetic properties to be controlled by an external magnetic field. For the position and orientation control of the microrobots, gradient magnetic field and/or constant magnetic field are used to generate magnetic force and magnetic torque. Therefore, precise magnetic field control is very important for accurate manipulation of the microrobot. MiniMag (MiniMag, Aeon Scientific GmbH, Switzerland) is an electromagnetic coil system to control the magnetic field on top of the system as shown in Fig. 2 [2232425]. The system has eight coils with two cameras for top and side views as shown in Fig. 2(a) and a transparent plastic container with silicon oil is used to provide the fluidic environment for the navigation of the microrobots as indicated by the red circle in Fig. 2(b). In this figure, a simple cylindrical magnet is used to demonstrate the control mechanism.
OctoMag (OctoMag, Aeon Scientific GmbH, Switzerland) is another magnetic control system with bigger working space, higher magnetic field, and higher magnetic field gradient compared to the MiniMag [2627]. OctoMag is shown in Fig. 3 and it has eight coils with cores for each coil. The output magnetic fields for MiniMag and OctoMag are ~ 20 mT and ~ 50 mT, respectively. The output magnetic field gradients for MiniMag and OctoMag are ~ 2 T/m and ~ 5 T/m, respectively. The maximum working spaces are roughly 10 mm3 and 20 mm3 for MiniMag and OctoMag, and both of the systems have 5 degrees of freedom: two orientations and 3 positions. The magnetic forces for MiniMag and OctoMag are 5.399*10-4 N and 1.349*10-3 N in the case of a cylindrical neodymium magnet (diameter: 0.5 mm, length: 1 mm).
Bio-scaffold is a porous structure which is used for regeneration of cells and/or tissues by cultivation of tissues on the structure [28293031]. For culturing cells or tissues using a scaffold, appropriate structure should be selected for the purposed target application. If the structure is planned to be used as a microrobot, the structure design is more important for optimal control and efficiency of movement. In addition, the shape of the target region and surrounding environment should be also considered before designing of the microrobot. Some of the microrobots are to remain in the body and some others could be dissolved by body fluids depending on the purpose of the microrobots. In both cases, the general purpose of the scaffold type microrobots is transporting tissues, cells or drug into a target area (2). Producing proper medium supply, uniform cell distribution and increasing cell density are important issues in 3D cell culture on a scaffold type microrobot to maintain the tissues in an environment with structural and functional complexity. The 3D porous microrobots fabricated by the 3D laser-lithography have the potential to transport multiple cells and/or drug at a time since the structures of the scaffold type microrobots can be easily customized for the size of the targeted cell or drug.
A scanning electron microscope (SEM) image of the fabricated scaffold type microrobots are shown in Fig. 4. The scale bars in Fig. 4 are ~ 50 µm and the size of a cubicle is about 10 µm3 and it can be optimized for the size of cell or drug to be transported [2]. The scaffold type microrobots can be wirelessly controlled by external magnetic fields using a magnetic control system. A series of captured images of a video are shown in Fig. 5 to show autonomous targeted control of the scaffold type microrobot by 13 mT magnetic field. The dark gray circles are the targeted points for autonomous movement of the scaffold type microrobot following the track made by the gray circles. The upper left insets in Fig. 5 show the electric currents for each coil at the given time during the autonomous targeted control of the microrobot.
Biomimetic is the approach that learns from nature to achieve an effective and environmentally friendly system [4323334]. For microrobots in small scale, microorganisms sometimes provide the ideas that show how to move at a small scale in a fluidic environment. Among the various motions of microorganisms, prokaryotic and eukaryotic flagella motion and ciliary motion are well understood [3435]. Prokaryotic and eukaryotic flagella show the corkscrew motion [416343637] and the traveling-wave motion [38394041], respectively, and the cilia moves by forward and backward stroke motions [842]. The key modality of these motions of microorganisms in the micro-scale environment is non-reciprocal motion.
Microorganisms without chirality should have non-time and axial-symmetric stroke motion to generate non-reciprocal motion [83839404142]. Microrobots with non-reciprocal motion by non-reciprocal actuation were not implemented before because of the difficulty of implementing non-symmetrical actuation and tiny flexible ciliary structures. Ciliary microrobots were designed, fabricated, and their position and orientation were manipulated by non-reciprocal actuation with stepping magnetic fields [84041]. The ciliary microrobots are implemented to mimic cilia based microorganisms such as paramecium. As shown in Fig. 6, the ciliary microrobot was fabricated by 3D laser-lithography system [282043], and nickel and titanium layers were deposited on the cilia part only by the sputtering system for magnetic manipulation and biocompatibility, respectively. Fig. 6(a) is the design of the paramecium inspired microrobot with a temporary cover to block the deposition of metal layers on the body of the ciliary microrobot. The fabricated ciliary microrobots with and without the mask structure are shown in Fig. 6(b). The fabricated microrobots were manipulated by magnetic manipulator which generates stepping magnetic fields (nonsymmetric on-off fields) to translate the cilia with nonaxial symmetric actuation forces and the cilia tend to align with the applied magnetic field direction [8]. The size of the microrobot is designed as 220 µm for body length, 60 µm for body diameter, and 4 by 10 by 75 µm for a cilium. The translational velocities of the ciliary microrobot were evaluated with different frequencies and magnetic field intensities. The maximum velocity of the ciliary microrobot was 340 µm/s which is achieved under the 9.5 mT of field intensity and 60 Hz of frequency with 110° of oscillating polar angle. The different stroke pattern during the power and recovery stroke generates the two different actuation forces which allows the microrobots to achieve the translational forward force. Fig. 7 explains the mechanism to generate propulsion force by stroke and recovery motions.
We reviewed the fabrication and control methods for scaffold type and ciliary microrobots for possible biomedical applications. The fabrication was done using negative photoresists such as SU-8, IP-Dip, and IP-L with a 3D laser-lithography system and a sputter. The 3D porous scaffold type microrobot was designed to contain many cells or drug for precise transportation. The fabrication parameters of the microrobot was optimized by making test structures with various laser intensities and writing speeds of the 3D laser-lithography system. The ciliary microrobot was created by the same fabrication method. Both the scaffold type and ciliary microrobots were manipulated by magnetic control systems. MiniMag and OctoMag were introduced and their specifications were compared. The fabricated scaffold type and ciliary microrobots were manipulated to show the possible applications of the microrobots for precise cell and drug delivery.
Figures and Tables
 | Fig. 1Fabrication process for 3D porous microrobot using a 3D laser lithography system. (a) Cleaning glass wafer using IPA, (b) Drop-cast of 50~100 ul negative photoresist, (c) laser writing, (d) development, (e) nickel and titanium deposition by a sputter, and (f) fabricated structures for parameter test. |
 | Fig. 2Magnetic field control system. (a) Overview of the system and (b) a closed view for a container with fluid to immerse a small magnet for control test. |
ACKNOWLEDGMENTS
We thank the CCRF of DGIST for technical support. Funding for this research was provided by National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NO. 2014R1A2A2A01006223) and Korea Evaluation Institute of Industrial Technology (KEIT) funded by the Ministry of Trade, Industry & Energy (NO. 10052980).
References
1. Grady MS, Howard MA, 3rd , Molloy JA, Ritter RC, Quate EG, Gillies GT. Nonlinear magnetic stereotaxis: three-dimensional, in vivo remote magnetic manipulation of a small object in canine brain. Med Phys. 1990; 17:405–415.

2. Kim S, Qiu F, Kim SH, Ghanbari A, Moon CI, Zhang L, et al. Fabrication and Characterization of Magnetic Microrobots for Three-Dimensional Cell Culture and Targeted Transportation. Adv Mater. 2013; 25:5863–5868.

3. Nelson BJ, Kaliakatsos IK, Abbott JJ. Microrobots for minimally invasive medicine. Annu Rev Biomed Eng. 2010; 12:55–85.

4. Peyer KE, Zhang L, Nelson BJ. Bio-inspired magnetic swimming microrobots for biomedical applications. Nanoscale. 2013; 5:1259–1272.

5. Bailly Y, Amirat Y, Fried G. Modeling and Control of a Continuum Style Microrobot for Endovascular Surgery. IEEE Trans Robot. 2011; 27:1024–1030.

6. Mhanna R, Qiu F, Zhang L, Ding Y, Sugihara K, Zenobi-Wong M, et al. Artificial bacterial flagella for remote-controlled targeted single-cell drug delivery. Small. 2014; 10:1953–1957.

7. Pawashe C, Floyd S, Sitti M. Modeling and Experimental Characterization of an Untethered Magnetic Micro-Robot. Int J Rob Res. 2009; 28:1077–1094.

8. Kim S, Lee S, Lee J, Nelson BJ, Zhang L, Choi H. Fabrication and Manipulation of Ciliary Microrobots with Non-reciprocal Magnetic Actuation. Sci Rep. 2016; 6.

9. Tottori S, Zhang L, Qiu F, Krawczyk KK, Franco-Obregon A, Nelson BJ. Magnetic helical micromachines: fabrication, controlled swimming, and cargo transport. Adv Mater. 2012; 24:811–816.

10. Temel FZ, Yesilyurt S. Confined swimming of bio-inspired microrobots in rectangular channels. Bioinspir Biomim. 2015; 10:016015.

11. Qiu FM, Fujita S, Mhanna R, Zhang L, Simona BR, Nelson BJ. Magnetic Helical Microswimmers Functionalized with Lipoplexes for Targeted Gene Delivery. Adv Funct Mater. 2015; 25:1666–1671.

13. Xiong W, Zhou YS, He XN, Gao Y, Mahjouri-Samani M, Jiang L, et al. Simultaneous additive and subtractive three-dimensional nanofabrication using integrated two-photon polymerization and multiphoton ablation. Light Sci Appl. 2012; 1.

14. Buckmann T, Stenger N, Kadic M, Kaschke J, Frolich A, Kennerknecht T, et al. Tailored 3D Mechanical Metamaterials Made by Dip-in Direct-Laser-Writing Optical Lithography. Adv Mater. 2012; 24:2710–2714.

15. Vollmers K, Frutiger DR, Kratochvil BE, Nelson BJ. Wireless resonant magnetic microactuator for untethered mobile microrobots. Appl Phys Lett. 2008; 92(14):

16. Zhang L, Abbott JJ, Dong LX, Kratochvil BE, Bell D, Nelson BJ. Artificial bacterial flagella: Fabrication and magnetic control. Appl Phys Lett. 2009; 94(6):

17. Khamesee MB, Kato N, Nomura Y, Nakamura T. Design and control of a microrobotic system using magnetic levitation. IEEE ASME Trans Mechatron. 2002; 7:1–14.

18. Yesin KB, Vollmers K, Nelson BJ. Modeling and control of untethered biomicrorobots in a fluidic environment using electromagnetic fields. Int J Robot Res. 2006; 25:527–536.

19. Thiel M, Fischer J, von Freymann G, Wegener M. Direct laser writing of three-dimensional submicron structures using a continuous-wave laser at 532 nm. Appl Phys Lett. 2010; 97(22):

20. Klein F, Richter B, Striebel T, Franz CM, von Freymann G, Wegener M, et al. Two-Component Polymer Scaffolds for Controlled Three-Dimensional Cell Culture. Adv Mater. 2011; 23:1341–1345.

21. Renner M, von Freymann G. Spatial correlations and optical properties in three-dimensional deterministic aperiodic structures. Sci Rep. 2015; 5.

22. Available from: http://www.nanoscribe.de/en/products/ip-photoresists.
23. Ghanbari A, Chang PH, Nelson BJ, Choi H. Magnetic actuation of a cylindrical microrobot using time-delay-estimation closed-loop control: modeling and experiments. Smart Mater Struct. 2014; 23(3):

24. Ghanbari A, Chang PH, Choi H, Nelson BJ. Time delay estimation for control of microrobots under uncertainties. In : 2013 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM); 2012; Wollongong, Australia.
25. Xu TT, Yu JF, Yan XH, Choi H, Zhang L. Magnetic Actuation Based Motion Control for Microrobots: An Overview. Micromachines (Basel). 2015; 6:1346–1364.

26. Kummer MP, Abbott JJ, Kratochvil BE, Borer R, Sengul A, Nelson BJ. OctoMag: An Electromagnetic System for 5-DOF Wireless Micromanipulation. IEEE Trans Robot. 2010; 26:1006–1017.

27. Bergeles C, Kratochvil BE, Nelson BJ. Visually Servoing Magnetic Intraocular Microdevices. IEEE Trans Robot. 2012; 28:798–809.

28. Tsang VL, Bhatia SN. Three-dimensional tissue fabrication. Adv Drug Deliv Rev. 2004; 56:1635–1647.

29. Subia B, Kundu J, Kundu SC. Biomaterial scaffold fabrication techniques for potential tissue engineering applications. In : Eberli D, editor. Tissue Engineering. 2010. p. 141–157.
30. Kapyla E, Aydogan DB, Virjula S, Vanhatupa S, Miettinen S, Hyttinen J, et al. Direct laser writing and geometrical analysis of scaffolds with designed pore architecture for three-dimensional cell culturing. J Micromech Microeng. 2012; 22(11):
31. Klein F, Striebel T, Fischer J, Jiang ZX, Franz CM, von Freymann G, et al. Elastic Fully Three-dimensional Microstructure Scaffolds for Cell Force Measurements. Adv Mater. 2010; 22(8):868–871.

32. Purcell EM. Life at Low Reynolds-Number. Am J Phys. 1977; 45:3–11.
33. Abbott JJ, Peyer KE, Lagomarsino MC, Zhang L, Dong LX, Kaliakatsos IK, et al. How Should Microrobots Swim? Int J Robot Res. 2009; 28:1434–1447.

34. Qiu FM, Zhang L, Peyer KE, Casarosa M, Franco-Obregon A, Choi H, et al. Noncytotoxic artificial bacterial flagella fabricated from biocompatible ORMOCOMP and iron coating. J Mater Chem B Mater Biol Med. 2014; 2:357–362.

35. Zhang L, Abbott JJ, Dong LX, Peyer KE, Kratochvil BE, Zhang HX, et al. Characterizing the Swimming Properties of Artificial Bacterial Flagella. Nano Lett. 2009; 9:3663–3667.

36. Gao W, Peng XM, Pei A, Kane CR, Tam R, Hennessy C, et al. Bioinspired Helical Microswimmers Based on Vascular Plants. Nano Lett. 2014; 14:305–310.

37. Peyer KE, Tottori S, Qiu F, Zhang L, Nelson BJ. Magnetic helical micromachines. Chemistry. 2013; 19:28–38.

38. Dreyfus R, Baudry J, Roper ML, Fermigier M, Stone HA, Bibette J. Microscopic artificial swimmers. Nature. 2005; 437:862–865.

39. Gao W, Sattayasamitsathit S, Manesh KM, Weihs D, Wang J. Magnetically powered flexible metal nanowire motors. J Am Chem Soc. 2010; 132:14403–14405.

40. Khalil IS, Dijkslag HC, Abelmann L, Misra S. MagnetoSperm: A microrobot that navigates using weak magnetic fields. Appl Phys Lett. 2014; 104(22):223701.

41. Jang B, Gutman E, Stucki N, Seitz BF, Wendel-Garcia PD, Newton T, et al. Undulatory Locomotion of Magnetic Multilink Nanoswimmers. Nano Lett. 2015; 15:4829–4833.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download