Abstract
Systemic infections that are caused by various types of pathogenic organisms can be spread to the eyes as well as to other solid organs. Bacteria, parasites, and viruses can invade the eyes via the bloodstream. Despite advances in the diagnosis and treatment of systemic infections, many patients still suffer from endogenous ocular infections; this is particularly due to an increase in the number of immunosuppressed patients such as those with human immunodeficiency virus infection, those who have had organ transplantations, and those being administered systemic chemotherapeutic and immunomodulating agents, which may increase the chance of ocular involvement. In this review, we clinically evaluated posterior segment manifestations in the eye caused by hematogenous penetration of systemic infections. We focused on the conditions that ophthalmologists encounter most often and that require cooperation with other medical specialists. Posterior segment manifestations and clinical characteristics of cytomegalovirus retinitis, endogenous endophthalmitis, toxoplasmosis, toxocariasis, and ocular syphilis are included in this brief review.
Ocular inflammation is often the first evidence of systemic infections. Despite the protection of the blood-retinal barrier, various pathogenic organisms can invade the eye via the bloodstream. Sources of immunosuppression affect not only a patient's susceptibility to ocular infection, but can also change the features of the disease. Posterior segment manifestations in the eye due to systemic infections have many disease presentations that cannot all be covered in this article. Therefore, we have described the most common ocular infections associated with systemic infections.
Cytomegalovirus (CMV) retinitis is the most common opportunistic ocular infection in immunosuppressed patients such as those with acquired immune deficiency syndrome (AIDS) and those on chemotherapeutic agents or immune modulators. CMV can invade to human in various routes such as placental transfer, sexual contact, or blood transfusion. Once infected, CMV remains in the body during one's lifetime. In immunocompetent individuals, CMV stays in a latent state. However, CMV is reactivated in hosts with immunosuppressed condition.
The most common cases of CMV retinitis happen in patients with AIDS, and CMV retinitis accounts for 80% of CMV-related diseases in AIDS patient [1]. Traditionally, it is well known that AIDS patients who have CD4 T-cell counts of less than 50 cells/mm3 are at increased risk of CMV infection or reactivation. Since the arrival of highly active anti-retroviral therapy (HAART), there has been a 75% reduction in the number of new cases of CMV retinitis compared with the pre-HAART era [2]. However, about 20% of those patients have poor response to HARRT, and they continue to be at high risk of developing CMV retinitis [3]. Moreover, as numbers of organ transplants have increased, post-transplant CMV retinitis rise the other significant complication despite increased and treatment.
The presenting symptoms of CMV retinitis are decreased vision, floaters, photopsia, eyeball pain, and scotomas. Floaters and photopsia in patients with AIDS are regarded as predictors of developing CMV retinitis [4]. Anterior segment examination usually reveals very little or mild inflammation, and may include keratic precipitates, anterior chamber inflammatory cells, and vitritis. Retinal findings are progressive necrotizing retinitis, which affect the posterior pole or peripheral retina, or both, and retinitis can be occurred unilateral or bilateral. The retinitis contains granular intraretinal lesions, retinal infiltrations, and necrotic lesions often develop along the vascular arcades in the posterior pole (Fig. 1A). There are three patterns of active CMV retinitis: hemorrhagic CMV retinitis, in which lesions with predominantly retinal hemorrhage with some necrosis ; brush fire shaped CMV retinitis, in which lesions with progressive expansion of yellow-white margin around retinal necrosis; and granular CMV retinitis, in which a less intense white granular lesions without or scanty retinal hemorrhage (Fig. 1B). The progression of retinitis has been defined as 1) the movement of the lesion border by 750 µm, or 2) the occurrence of new retinal lesion that is at least a quarter of the disc area in size and at least 750 µm away from previous lesions [5]. Due to CMV retinitis lead to full-thickness retinal necrosis, retinal detachment can occur. Although there is 60% of reduction in the incidence of retinal detachment in post-HAART era, retinal detachment can cause a poor visual outcome in patients with CMV retinitis [6]. The risk of retinal detachment is increased if the lesions are large in size and anteriorly positioned. Optic disc, retina and iris neovascularization can occur. Optic nerve involvement has been reported up to 4% of CMV retinitis patients. In one retrospective study in Korea, CMV retinitis was common in patients with immunodeficient conditions including AIDS, cytotoxic chemotherapy, and immunosuppressive treatments. Thirty four percent of patients had bilateral disease. Retinitis and atrophic changes involving the macula were the major causes of visual loss. Although CMV retinitis was treated with antiviral agents in all cases, cataracts, cystoid macular edema, retinal detachment, epiretinal membrane, and immune recovery uveitis developed as late complications [7].
The diagnosis of CMV retinitis is based on ophthalmologic examinations, careful patient history, and positive results on serologic testing. Assays for the detection of CMV antigenemia are a simple and rapid test determining patients with CMV retinitis in one eye who are at the highest risk of developing CMV retinitis of the other eye. A positive polymerase chain reaction (PCR) result may be useful for the clinical diagnosis and monitoring the response to antiviral treatment. Detection of CMV DNA in ocular specimens by PCR is increasingly being performed. PCR regards as a sensitive and specific diagnostic test of CMV retinitis. CMV retinitis should be differentiated from other necrotizing retinitis such as acute retinal necrosis (ARN) or progressive outer retinal necrosis (PORN). ARN and PORN show more rapid progression than CMV retinitis. ARN tends to occur in immunocompetent patients, whereas PORN usually occurs in patients with advanced AIDS. Ocular syphilis and toxoplasmosis can also present as necrotizing retinitis in patients with AIDS.
The treatment goal of CMV retinitis is to preserve vision, prevent retinitis progression, and protect the previously uninfected eye. Treatment of CMV retinitis divides of two phases (induction and maintenance phase). During the induction phase, treatment is usually given over 2-3 weeks. In the maintenance phase, the drug dosage is reduced and maintained for several months. Many useful antiviral agents have been induced for the treatment of CMV retinitis; ganciclovir is the most commonly used antiviral agent. The intravenous (IV) induction protocol uses 5 mg/kg/ b.i.d for 2-3 weeks, followed by 5 mg/kg/ q.d in the maintenance phase. Intravitreal ganciclovir may also be effective in patients for whom intravenous ganciclovir has previously failed or in patients who need a higher concentration of drug in the eye. This may also avoid most systemic side effects of ganciclovir. Intravitreal injections are given at 0.2-2.0 mg/ twice per week during the induction phase, followed by weekly injections in the maintenance phase. Oral ganciclovir can be used during the maintenance phase of CMV retinitis with administration of 3-6 g/day [8]. Valganciclovir is another effective antiviral agent, which has been orally administered during the induction phase (900 mg/b.i.d) and 900 mg/q.d for the maintenance treatment of CMV retinitis [9]. It is effective and convenient treatment for patients, recently, oral valganciclovir is gaining in popularity in clinical settings. Foscarnet and cidofovir are other treatment options of CMV retinitis. The induction dose of foscarnet is 90 mg/kg/b.i.d for 2-3 weeks for the induction phase followed by administration 90 mg/kg/q.d for maintenance treatment. However, foscarnet has serious side effect such as nephrotoxicity. Intravitreal foscarnet can be given at a dose of 2.4 mg/ once or twice weekly. Standard dosing of cidofovir is 5 mg/kg administered IV every week for 2 weeks during the induction phase, followed by biweekly IV administration of 3-5 mg/kg. For the treatment of CMV-associated retinal detachment, the standard surgical approach is vitrectomy with silicone oil tamponade due to the presence of multiple, ill-defined retinal breaks. Visual prognosis is often poor owing to optic atrophy that results from widespread disease.
Although the prevalence of CMV retinitis has decreased over the past 20 years as a result of the use of HAART in AIDS patients, some patients who have intolerance to antiretroviral treatment still remain at high risk for developing CMV retinitis. In addition, increase in number of bone-marrow and organ transplantation and use of immunosuppressive therapies raise the likelihood of CMV retinitis in non-AIDS patients. CMV retinitis has a highly characteristic retinal appearance, but the lesions vary among patients. Therefore, it is important to maintain a high index of suspicion and closer ophthalmologic examination in potential CMV infections.
Endophthalmitis is defined as infection of the eye involving the vitreous and/or aqueous fluids. Endogenous endophthalmitis occurs as a result of hematogenous transmission of microbial organisms inside the eye. It accounts for approximately 2-8% of all endophthalmitis cases and remains a serious cause of intraocular inflammation. Endogenous endophthalmitis can occur during systemic bacteremia or fungemia. Systemic infections are misdiagnosed up to 50% of cases, especially if it is the first sign of systemic disease [10]. Patients with endogenous endophthalmitis are typically systemically deteriorated, but endophthalmitis can occur in apparently healthy patients. Clinicians must bear this disease in mind if it is to be correctly diagnosed. Generally, ophthalmologist should consult with internist to perform the medical evaluation. Bilateral infection occurs in 15-25% of cases. Although most patients may have a known infection focus, the specific origin of the infection is not always identified. Jackson et al. reported the rate of unknown origin was over 40% [10]. They presumed that the negative systemic origin came from a transient fungemia or bacteremia.
Gram-positive bacteria are the most common cause of endogenous bacterial endophthalmitis; streptococcal species are the commonest organism and Staphylococcus aureus is the most common individual organism. However, recently, with an increase in the incidence of Klebsiella species, gram-negative bacterial infections have accounted for a greater proportion of reported endogenous endophthalmitis cases. About 80% of these Klebsiella infections were reported from East Asia due to high incidence of hepatobiliary infection such as liver abscess [1112]. Known predisposing factors of endogenous bacterial endophthalmitis are infectious endocarditis, urinary tract infection, hepatobiliary infection, meningitis, wound infection, skin infection, and pulmonary infection. In a 15-year study of endogenous endophthalmitis in our center [13], diabetes and liver cirrhosis were the most common systemic problems. Klebsiella pneumoniae (48%) was the most common causative organism, although more recently gram-positive cocci have increased in frequency. Initial visual acuity greater than counting fingers, early vitrectomy, and gram positive cocci were significantly related to successful visual outcomes after the treatment of endophthalmitis in our study [13].
Common presenting symptoms of endogenous bacterial endophthalmitis include decreased visual acuity, floaters, mucous discharge, and eyeball pain. Ophthalmologic examinations often reveal anterior chamber hypopyon, septic emboli in retinal vessels, Roth spots, chorioretinal infiltrations and moderate to severe vitritis (Fig. 2).
The gold standard of diagnosis remains positive culture result. Anterior chamber fluid culture may be useful for patients with predominantly anterior segment involvement, but vitreous culture yields more positive result [10]. Repeated cultures may also be required if endophthalmitis is strongly suspected but initial results were negative. And once a diagnosis of endophthalmitis is confirmed, other organ involvement must be examined. As we mentioned above, these examinations are usually performed in consultation with an internist. A previous study reported that cultures were diagnostic in 96% of cases of endogenous bacterial endophthalmitis [14]. This result was inclusive of samples collected from multiple culture sites. The positive rates of cultures were 74% for vitreous fluids and 72% for blood culture.
The management of endogenous bacterial endophthalmitis depends on ophthalmological severity, the causative organism isolated. Most patients with endogenous bacterial endophthalmitis are necessary treatment with systemic antibiotics depending on the extent of the infection and the causative organisms and if vitreous seeding or macular-threatening disease is present, the injection of intravitreal antibiotics injection should be performed. The antibiotic used for empirical treatment should encompass a broad range of gram-positive and gram-negative bacteria. Intravitreal antibiotics have shown efficacy in cases of endogenous bacterial endophthalmitis. Vancomycin (1.0 mg/0.1 mL) is typically given for coverage against gram-positive organisms. Either ceftazidime (2.25 mg/0.1 mL) or amikacin (0.4 mg/0.1 mL) may be used to encompass gram-negative organisms [11]. Intravitreal antibiotic injections should be followed by topical antibiotics. Topical vancomycin (50 mg/mL) in combination with an amikacin (9 or 14 mg/mL) or ceftazidime (50 mg/mL) administered hourly is recommended. Vitrectomy has been used with variable results for the treatment of endogenous bacterial endophthalmitis. The advantages of vitrectomy for endogenous endophthalmitis include obtaining a sufficient vitreous sample and debulking of the vitreous cavity, allowing for the removal of the majority of infectious organisms and other inflammatory mediators. Moreover, the vitrectomy allows for improved drug circulation throughout the vitreous cavity. Jackson et al. [11] reported that this was a significant benefit to patients with endogenous bacterial endophthalmitis who were undergoing pars plana vitrectomy. However, in their review, the visual outcomes for patients were still poor. Eighty percent of eyes had only light perception vision or worse, and 25% of eyes required sacrificing the eye.
Fungal origins account for more than fifty percent of endogenous endophthalmitis cases. Candida albicans, followed by Aspergillus species, is the most common fungal organism [15]. Some epidemiological studies have reported that Candida species represent 35% of all cases of endogenous endophthalmitis, and its incidence in endogenous fungal endophthalmitis is reported at 56%, followed by Aspergillus at 24% [1016]. Our center has reported on fungal endophthalmitis over seven years. In a total 40 eyes from 30 patients with fungal endophthalmitis, Candida species were the most frequent causative organisms (88%). Visual prognosis was best in patients who had prompt vitrectomies and intravitreal injections of antifungal agents [17].
Known predisposing factors of endogenous fungal endopthalmitis are an indwelling central venous catheter, total intravenous nutrition, broad-spectrum antibiotics, glucocorticoid therapy, and recent abdominal surgery. The highly vascular choroid is seeded first, so the initial ophthalmologic manifestation is usually chorioretinitis with sparing vitreous. Chorioretinal lesions represent deep, focal, and white infiltrations, subretinal lesions, vitritis, retinal hemorrhage and cotton-wool spots (Fig. 3). Bilateral involvement is more common in endogenous fungal endophthalmitis than in endogenous bacterial endophthalmitis [1416]. Patients with early chorioretinitis may have no specific symptoms, unless the lesions are near the macula. As the infection advances, worsening of chorioretinitis and vitritis develops, and patients present floaters or decreased visual acuity. When inflammation spread to the aqueous humor and anterior segment of the eye, eyeball pain may also present as a symptom. Therefore, in patients with fungemia, funduscopic examination should be repeated after two weeks even in patients with a negative initial screening ophthalmoscopic examination [18].
The diagnosis of endogenous fungal endophthalmitis is mainly made based on ophthalmologic examinations. Vitreous culture is used as confirm diagnosis, but usually not necessary in patients who have known fungemia and fundus examination that is compatible with characteristic chorioretinitis. For patients with systemic fungal infection, successful culture results have been reported in 45-70% of cases with clinical findings of endogenous fungal endophthalmitis [19]. Clearly, suspicion of endogenous fungal endophthalmitis should be maintained even in the negative culture result for the diagnosis.
If patients present only chorioretinitis without vitreous seeding, they can be managed with systemic antifungal agents alone [20]. Fluconazole, voriconazole, and flucytosine have been used with good ocular penetration, but amphotericin B and the echinocandin of antifungal agents do not [21]. Systemic fluconazole should be continued for 2-4 weeks for the treatment of endogenous fungal endopthalmitis. Systemic flucytosine is used with good intraocular penetration but Candida species show high rates of resistance. Recently, voriconazole is the most commonly used antifungal agent because its higher affinity for the fungal enzymes that contributes to the construction of cell membranes. It may be used orally or intravenously at 200 mg/b.i.d [22]. Voriconazole is generally effective against most Candida species, Aspergillus species. Patients should be monitored with serial ophthalmoscopic examinations, and the dosage of antifungal agents should be adjusted accordingly. Intravitreal injections are necessary if there are macular-threatening lesions or serious vitreous seeding. Intravitreal voriconazole (100 µg) or amphotericin B (5-10 µg) can be used. If patients are refractory to systemic and/or intravitreal antifungal therapy, vitrectomy should not be delayed. Patients with endogenous fungal endophthalmitis have a better visual outcome than those with endogenous bacterial endophthalmitis. However, infection with Aspergillus species presents rapid progression, and the visual outcomes tend to be poor than candida or bacterial infections. Therefore, intravitreal injection of antifungal agents is recommended for cases of endogenous Aspergillus endophthalmitis [16].
Although the incidence of this disease remains low, endogenous endophthalmitis is an important disease for both ophthalmologists and internists. It is critical to initiate systemic antibiotics to treat the source of infection. For patients without a known source of systemic infection, clinicians should perform culture from multiple sites to find the origin of infection. In patients with highly suspected endogenous endophthalmitis, it is important to cooperate with a specialist of infectious disease to search for causative organisms or other organ involvement. Patients with characteristic chorioretinitis and with no or minimal vitritis may be initially managed with systemic antibiotics or antifungals and close observation. If treatment response to systemic therapy is poor, vitrectomy and intravitreal therapy should not be delayed. Using a high index of suspicion of endogenous endophthalmitis and timely treatment can reduce visual loss in this horrible disease.
Toxoplasma gondii is an obligate, intracellular, protozoan parasite that infects as a zoonotic pathogen. More than 30% of humans worldwide are estimated to be chronically infected with T. gondii [23]. However, seroprevalence varies greatly between different countries (20-70%) and even within countries. Lim et al. [24] reported the prevalence of T. gondii in 98 Korean patients with uveitis. Only 6 patients (6%) were seropositive for T. gondii in their analysis. In another study from Korea, the seroprevalence of T. gondii infection in Seoul and the island of Jeju-do were 8.0% and 11.3%, respectively [25]. Although ocular toxoplasmosis is not a common cause of posterior uveitis in some countries including Korea, it is one of the most common types (30-50%) of posterior uveitis in worldwide [26].
T. gondii exists in different morphologic and metabolic stages: oocysts are the product of the parasite's sexual cycle in the intestine of all felidae (cat family) and release infectious sporozoites. Tachyzoites are asexual forms, which replicate rapidly, enter all nucleated cells by active penetration, and form intracytoplasmic vacuoles. The tachyzoite causes a strong inflammatory response in the human body, and is therefore responsible for the clinical manifestations of the disease. Tissue cysts, which contain bradyzoites, represent the dormant stage of the parasite in tissues that are under pressure from the immune system [27]. They are remained in the brain, muscles and retina. Humans can get infected from consuming unheated meat or by sporulated oocysts containing water, sand, or vegetables. A recent Korean study reported that half of ocular toxoplasmosis patients had a history of consuming wild meat or deer blood [28]. After ingestion, cysts are disrupted and the bradyzoites are released into the intestinal lumen, where they rapidly enter cells and multiply as tachyzoites. Immunodeficiency allows reactivated parasites to proliferate and cause severe disease, whereas re-infection does not appear to cause clinically apparent disease [29]. Ocular toxoplasmosis was considered to be the result of a recurrence of the congenital toxoplasmosis. However, more recent reports suggest that acquired infection is a more common cause of ocular toxoplasmosis [23].
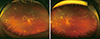
The presenting symptoms of ocular toxoplasmosis are decreased vision and floaters. The retina is the primary focus of toxoplamosis in the eye. Therefore, active lesions of ocular toxoplasmosis typically present with a focal necrotizing retinitis or retinochoroiditis (Fig. 4A). The hallmark features are necrotizing retinochoroiditis and satellite lesions adjacent to old hyperpigmented scars, as reactivation occurs at the borders of or close to scars (Fig. 4B). More than 70% of patients presenting to an ophthalmologist already have a combination of an active lesion and healed chorioretinal scar. This may imply that previous peripheral retinitis went unnoticed in these patients [30]. Active lesions are accompanied by various degrees of vitritis. In cases of intense vitritis, a bright white reflex is observable when the light of an indirect ophthalmoscope in shone into the back of the eye ("headlight in the fog"). Wide ranging anterior uveitis may be observed with either granulomatous or non-granulomatous inflammation. Optic nerve involvement has also been reported [31]. The first episode of ocular toxoplasmosis typically occurs in the second decade of life, the 5-year recurrence rate is 80%.
The diagnosis of ocular toxoplasmosis is made by clinically and based on ophthalmologic examination, and the presence of antibodies for T. gondii. When these clinical and serologic diagnosis is imprecise, detection of T. gondii antibody in ocular fluids or amplification of T. gondii DNA using PCR have been used successfully to confirm the diagnosis [32]. Several diagnostic test systems can be used either for screening purposes or for the discrimination of T. gondii-specific IgM, IgA, and IgG antibodies. In most cases, the presence of specific IgM and/or IgA antibodies in the serum indicates acute infection, whereas high-avidity IgG antibodies indicate previous infection [33].
There are many debates surrounding the treatment strategies of ocular toxoplasmosis. The anti-toxoplasmic agents do not eradicate tissue cysts and cannot prevent chronic infection. There are no treatments that have been proven to be more effective than the absence of treatment. Due to the fact that most cases of ocular toxoplasmosis are self-limiting, clinical indications for the treatment for ocular toxoplasmosis include lesions within the major vascular arcades of the retina, lesions close to the optic nerve, large lesions (>2 optic disc diameters), and retinochoroiditis in immunosuppressed patients, because untreated patients often develop fulminant, progressive lesions. There are many treatment options for ocular toxoplasmosis, but management using pyrimethamine, sulfadiazine and corticosteroids continues to be the most widely used method and is regarded as the "classical triple therapy" [2734]. Corticosteroids are often beneficial for reducing the tissue damage that is caused by the inflammatory response to tachyzoites. Recently, treatment with trimethoprim/sulfamethoxazole and oral corticosteroids has become an alternative option. This treatment had similar efficacy, fewer side effects, and better patient compliance than classical therapy in a randomized clinical trial [35]. Systemic clindamycin, azithromycin, and atovaquone have been introduced into clinical use but have not gained widespread acceptance [27]. Another treatment option is intravitreal injections of clindamycin (1.5 mg/0.1 mL) and dexamethasone (400 mg/0.1 mL) every 4 weeks. Intravitreal injection may be useful option for patients such as pregnant women or children who cannot tolerate systemic anti-toxoplasmic agents [35].
The complications of ocular toxoplasmosis include chronic anterior uveitis, cataract, glaucoma, corneal decompensation, retinal detachment, macular edema and optic nerve atrophy. Subretinal neovascularization and other retinal vascular lesions such as branch artery occlusion, phlebitis, and scleritis have been reported as late complications of ocular toxoplasmosis.
Ocular toxoplasmosis is an important cause of infectious posterior uveitis that is mainly acquired postnatally. Proper diagnosis relies on typical ophthalmological manifestations and many therapeutic options are available. A better clinical and pathogenic understanding could lead to more effective management to prevent this common cause of visual loss.
Toxocara is a species of nematode for which the hosts are the dog (Toxocara canis) and cat (Toxocara cati). Humans are infected through ingestion of larvae in contaminated soil, fecal-oral transmission, or through food products. Most infected larvae of Toxocara remained in the liver, but some migrate by hematogenous spread and infect the brain, lungs, muscles, and eye. In these sites of infection, the larvae do not develop into mature worms but may still cause a severe inflammatory reaction. Ocular toxocariasis is an important international cause of infectious posterior uveitis. Chang et al. [36] reported that ocular toxocariasis was the fifth most common cause of posterior uveitis in their meta-analysis. The incidence varies significantly with geographic region and is most common in the southeastern America, Argentina, and Japan. Lim et al. [24] reported that 23% of 98 Korean patients with uveitis were positive for serum IgG against T. canis.
Clinically, human toxocariasis comprises visceral larva migrans and ocular larva migrans. Visceral larva migrans is the systemic toxocariasis and is characterized by flu-like symptoms, hepatosplenomegaly and rash. Ocular larva migrans is the term of ocular toxocariasis. There are four types of manifestations in ocular toxocariasis: chronic endophthalmitis, posterior pole granuloma, peripheral granuloma, and atypical ocular toxocariasis. About fifty percent of all cases of ocular toxocariasis show peripheral granuloma, and posterior pole granuloma and chronic endophthalmitis constitute a quarter of cases, respectively [37]. Retinal lesions are characterized by a white granulomatous mass in posterior pole or peripheral retina (Fig. 5). Vitreous inflammation may or may not be accompanied. Tractional retinal folds may be present between the granuloma and macula or optic disc. Less common manifestations include endophthalmitis, mild anterior chamber inflammation, and cataract. Peripheral disease has been reported a worse clinical outcome than posterior pole disease. Although the average age of ocular toxocariasis is around 8 years, the age at presentation may up to 30 years. Presenting symptoms are painless visual loss, strabismus, or leukocoria, and signs and symptoms nearly always occur unilaterally. Therefore, ocular toxocariasis should be differentiated with congenital cataract, retinoblastoma, Coats' disease, retinopathy of prematurity (ROP), persistent hyperplastic primary vitreous (PHPV), and familial exudative vitreoretinopathy (FEVR) [38].
The presumed diagnosis of ocular toxocariasis is made based on characteristic localized granuloma in many cases. Supported diagnosis of ocular toxocariasis is made serologically with a test for IgG antibodies to Toxocara. However, considering serological studies of ocular toxocariasis have a nature of high false positive rate, there is no reliable in vivo gold standard to confirm the presence of Toxocara larvae. A definitive diagnosis can be made by enzyme-linked immunosorbent assay testing of specimens obtained from an anterior chamber or vitreous [39].
The effectiveness of systemic antihelminthic therapy for ocular toxocariasis is still unclear. Ophthalmologists should decide when and how therapeutic approaches should be used, as ocular toxocariasis may be self-limiting. If the sight threatening lesions develop from the infection, intervention is usually mandated. Albendazole 10-15 mg/kg/day or thiabendazole 25 mg/kg/day for 14 days can be used [40]. Corticosteroids are used to prevent the development of inflammatory tractional folds. Topical and periocular steroids have also been used in cases of anterior chamber inflammation. In these cases, cycloplegic eye drops may also be used to prevent anterior and posterior synechiae. Retinal detachment is the most common indication for vitrectomy in ocular toxocariasis. Some investigators have reported that an Nd:YAG laser was used to direct photodestruction of the larvae [41]. Antivascular endothelial growth factor therapy has been used to treat the rare complication of juxtafoveal choroidal neovascularization [42].
The most important point to the management of ocular toxocariasis is prevention, such as education programs to decrease the risk of ingestion of Toxocara larvae, may lead to a reduced incidence of ocular toxocariasis.
Infection with the spirochetal bacterium Treponema pallidum causes systemic and ocular syphilis, a disease that affects most organs and that historically was a common cause of ocular inflammation. There are an estimated 12 million new syphilis cases every year worldwide, over 90% of which occur in developing countries. Increases in the reported cases occur most commonly in men who have sex with men and those who are coinfected with HIV [4344]. HIV alters the clinical severity of syphilis and increases the syphilitic central nervous system involvement. In syphilis patients with HIV infection who are not receiving HARRT, ocular syphilis is more frequently bilateral and more commonly involves the posterior segment.
Ocular involvement of syphilis occurs in stages in which the organism has spread hematogenously such as secondary, latent, and tertiary syphilis. Clinical manifestations of ocular syphilis are variable, and the disease has a feature of mimicking many other ocular diseases.
Ocular syphilis can present as a nonspecific anterior, intermediate, posterior, or panuveitis occurring 2.5-5% of patients with tertiary syphilis. Episcleritis/scleritis, keratitis, iris nodules, and posterior synechiae can also occur. In a 5-year observational study in Korea, ocular syphilis manifested as nongranulomatous anterior uveitis in half of all cases. Posterior segment findings included vitritis (84%), retinal vasculitis (58%), and chorioretinitis (27%) [45]. Retinal lesions include the presence of superficial retinal precipitates in syphilitic panuveitis. The other distinctive pattern is that of acute syphilitic posterior placoid chorioretinitis, which occur mostly in immunocompromised patients and is characterized by placoid yellowish or gray lesions with faded centers at the level of the retinal pigment epithelium accompanied by vitritis [46]. Other manifestations include vitreous inflammation, retinal vasculitis (Fig. 6), retinal vein occlusion, retinal detachment, and necrotizing chorioretinitis. Optic nerve findings include optic disc edema, neuroretinitis, and optic nerve gumma.
The diagnosis of ocular syphilis is made based on ophthalmologic examination, and confirmative serologic test. In AIDS patients, the presentation of ocular syphilis may be atypical, and so a strong clinical awareness is important when evaluating these patients. For most cases of uveitis with unknown origin, the strict rule of always placing syphilis in the differential diagnosis and performing serological testing is important. Serologic testing with nontreponemal and treponemal tests is most commonly used in ocular syphilis as well as systemic syphilis. It is important to do both treponemal and nontreponemal tests. There is a significant proportion of patients with either early or late syphilis who will be positive by treponemal-specific tests and negative by RPR. Therefore, currently recommended methods are immunoassays to detect antibodies to treponemal antigens, followed by nontreponemal test such as rapid plasma reagin (RPR) test [47]. If the serologic tests are positive by enzyme immunoassay or chemiluminescent immunoassay and negative on RPR are performed for a confirmatory T. pallidum particle agglutination (TPPA) test, and if that test is positive, a confirmative diagnosis of syphilis is considered. The RPR test is also useful as an indicator of response to treatment.
If positive syphilis serology is found, clinicians should also do additional test for the diagnosis of HIV. Furthermore, patients in any stage of syphilis with unexplained ophthalmic manifestations are needed further investigation with examination of the cerebrospinal fluid. Patients need to contact previous sexual partners so that they can be clinically evaluated and treated.
Ocular syphilis is considered to be a secondary syphilis and a neurosyphilis. The Centers for Disease Control (CDC) recommended that ocular syphilis should be treated in the same manner as neurosyphilis [4849] and intravenous penicillin is the treatment of choice. When a course of penicillin G is completed, three doses of benzathine penicillin by weekly intramuscular injection may be administered as an extra measure, but is not a substitute for initial therapy. A subsequent four-fold decrease in titer by the same nontreponemal test after treatment represents evidence of a response to treatment. Ceftriaxone and azithromycin may be an effective treatment options, until resistance was determined in several isolates [50]. Corticosteroids are generally not used, but could be considered for the treatment of inflammatory complications such as macular edema. Clinically, the inflammation subsides with penicillin treatment and visual improvement occurs within one month [46].
Ocular syphilis remains an important cause of ocular inflammatory disease. Without sufficient treatment, it is associated with poor visual outcome and high risk of both systemic and ocular complications. Nonetheless, if it was diagnosed promptly, it may be curable with a relatively short course of antibiotic treatment.
Systemic infections can be the causes of disease in distant organs including the eyes by a variety of ways. So, diagnosis and decision of treatment for ocular infection may be challenging. In addition, in some patients with serious systemic conditions, ocular symptoms can go unnoticed if they were not suspected by physicians, resulting in an irreversible visual loss later on.
Considering these possible and serious consequences, an interdisciplinary approach by ophthalmologists and physicians to ocular infection is essential. Also, in some circumstances, ocular symptoms of endogenous endophthalmitis become the presenting feature of systemic infection, such as a liver abscess or infectious endocarditis. Therefore, knowledge of these complications by physicians is crucial for the prompt diagnosis and decision of treatment.
In conclusion, when patients present with suspicious ocular conditions to ophthalmologists or medical specialists should keep in mind that the underlying systemic infection could be the cause of secondary ocular infection.
Figures and Tables
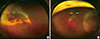 | Fig. 1Necrotizing retinitis of CMV retinitis. (A) mixed granular and hemorrhagic retinitis, (B) granular retinitis. |
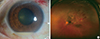 | Fig. 2Endogenous Klebsiella endophthalmitis. (A) anterior chamber hypopyon, (B) marked chorioretinitis and retinal hemorrhage with vitritis. |
 | Fig. 4Chorioretinal lesions of ocular toxoplasmosis. (A) active necrotizing retinochoroiditis with moderate vitritis, (B) healed active lesion with hyperpigmented scar of ocular toxoplasmosis. |
References
1. Gallant JE, Moore RD, Richman DD, Keruly J, Chaisson RE. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. The Zidovudine Epidemiology Study Group. J Infect Dis. 1992; 166:1223–1227.

2. Jacobson MA, Stanley H, Holtzer C, Margolis TP, Cunningham ET. Natural history and outcome of new AIDS-related cytomegalovirus retinitis diagnosed in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000; 30:231–233.

3. Le Moing V, Chene G, Carrieri MP, Alioum A, Brun-Vezinet F, Piroth L, et al. Predictors of virological rebound in HIV-1-infected patients initiating a protease inhibitor-containing regimen. Aids. 2002; 16:21–29.

4. Hodge WG, Boivin JF, Shapiro SH, Lalonde RG, Shah KC, Murphy BD, et al. Clinical risk factors for cytomegalovirus retinitis in patients with AIDS. Ophthalmology. 2004; 111:1326–1333.

5. Studies of ocular complications of AIDS Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial: 1. Rationale, design, and methods. AIDS Clinical Trials Group (ACTG). Control Clin Trials. 1992; 13:22–39.
6. Jabs DA, Van Natta ML, Thorne JE, Weinberg DV, Meredith TA, Kuppermann BD, et al. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 2. Second eye involvement and retinal detachment. Ophthalmology. 2004; 111:2232–2239.

7. Kwun YK, Chae JB, Ham DI. Cinical manifestations and prognosis of cytomegalovirus retinitis. J Korean Ophthalmol Soc. 2010; 51:203–209.

8. Lalezari JP, Friedberg DN, Bissett J, Giordano MF, Hardy WD, Drew WL, et al. High dose oral ganciclovir treatment for cytomegalovirus retinitis. J Clin Virol. 2002; 24:67–77.

9. Segarra-Newnham M, Salazar MI. Valganciclovir: a new oral alternative for cytomegalovirus retinitis in human immunodeficiency virus-seropositive individuals. Pharmacotherapy. 2002; 22:1124–1128.

10. Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore). 2003; 82:97–105.
11. Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: a 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003; 48:403–423.

12. Jackson TL, Paraskevopoulos T, Georgalas I. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv Ophthalmol. 2014; 59:627–635.

13. Lee S, Um T, Joe SG, Hwang JU, Kim JG, Yoon YH, et al. Changes in the clinical features and prognostic factors of endogenous endophthalmitis: fifteen years of clinical experience in Korea. Retina. 2012; 32:977–984.

14. Okada AA, Johnson RP, Liles WC, D'Amico DJ, Baker AS. Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology. 1994; 101:832–838.
15. Smith SR, Kroll AJ, Lou PL, Ryan EA. Endogenous bacterial and fungal endophthalmitis. Int Ophthalmol Clin. 2007; 47:173–183.

16. Schiedler V, Scott IU, Flynn HW Jr, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol. 2004; 137:725–731.

17. Kim DY, Moon HI, Joe SG, Kim JG, Yoon YH, Lee JY. Recent Clinical Manifestation and Prognosis of Fungal Endophthalmitis: a 7-year experience at a tertiary referral center in Korea. J Korean Med Sci. 2015; 30:960–964.

18. Krishna R, Amuh D, Lowder CY, Gordon SM, Adal KA, Hall G. Should all patients with candidaemia have an ophthalmic examination to rule out ocular candidiasis? Eye (Lond). 2000; 14(Pt 1):30–34.

19. Anand A, Madhavan H, Neelam V, Lily T. Use of polymerase chain reaction in the diagnosis of fungal endophthalmitis. Ophthalmology. 2001; 108:326–330.

20. Schwartz SG, Davis JL, Flynn HW. Endogenous Endophthalmitis. In : Ryan SJ, editor. Retina. 5th ed. 2013.
21. Riddell Jt, Comer GM, Kauffman CA. Treatment of endogenous fungal endophthalmitis: focus on new antifungal agents. Clin Infect Dis. 2011; 52:648–653.

22. Breit SM, Hariprasad SM, Mieler WF, Shah GK, Mills MD, Grand MG. Management of endogenous fungal endophthalmitis with voriconazole and caspofungin. Am J Ophthalmol. 2005; 139:135–140.

23. Subauste CS, Ajzenberg D, Kijlstra A. Review of the series "Disease of the year 2011: toxoplasmosis" pathophysiology of toxoplasmosis. Ocul Immunol Inflamm. 2011; 19:297–306.

24. Lim SJ, Lee SE, Kim SH, Hong SH, You YS, Kwon OW, et al. Prevalence of Toxoplasma gondii and Toxocara canis among patients with uveitis. Ocul Immunol Inflamm. 2015; 23:111–117.

25. Lim H, Lee SE, Jung BK, Kim MK, Lee MY, Nam HW, et al. Serologic survey of toxoplasmosis in Seoul and Jeju-do, and a brief review of its seroprevalence in Korea. Korean J Parasitol. 2012; 50:287–293.

26. Park YH, Nam HW. Clinical features and treatment of ocular toxoplasmosis. Korean J Parasitol. 2013; 51:393–399.

27. Maenz M, Schluter D, Liesenfeld O, Schares G, Gross U, Pleyer U. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retin Eye Res. 2014; 39:77–106.

28. Park YH, Han JH, Nam HW. Clinical features of ocular toxoplasmosis in Korean patients. Korean J Parasitol. 2011; 49:167–171.

29. Elbez-Rubinstein A, Ajzenberg D, Darde ML, Cohen R, Dumetre A, Yera H, et al. Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. J Infect Dis. 2009; 199:280–285.

30. Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology. 2002; 109:869–878.
31. Eckert GU, Melamed J, Menegaz B. Optic nerve changes in ocular toxoplasmosis. Eye (Lond). 2007; 21:746–751.

32. Montoya JG, Parmley S, Liesenfeld O, Jaffe GJ, Remington JS. Use of the polymerase chain reaction for diagnosis of ocular toxoplasmosis. Ophthalmology. 1999; 106:1554–1563.

33. Marcolino PT, Silva DA, Leser PG, Camargo ME, Mineo JR. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by Western blotting. Clin Diagn Lab Immunol. 2000; 7:384–389.

34. Belfort R, Silveira C, Muccioli C. Ocular toxoplasmosis. In : Ryan SJ, editor. Retina. 5th ed. 2013.
35. Soheilian M, Sadoughi MM, Ghajarnia M, Dehghan MH, Yazdani S, Behboudi H, et al. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology. 2005; 112:1876–1882.

37. Stewart JM, Cubillan LD, Cunningham ET, Jr . Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005; 25:1005–1013.

38. Ament CS, Young LH. Ocular manifestations of helminthic infections: onchocersiasis, cysticercosis, toxocariasis, and diffuse unilateral subacute neuroretinitis. Int Ophthalmol Clin. 2006; 46:1–10.

39. Yokoi K, Goto H, Sakai J, Usui M. Clinical features of ocular toxocariasis in Japan. Ocul Immunol Inflamm. 2003; 11:269–275.

40. Schneier AJ, Durand ML. Ocular toxocariasis: advances in diagnosis and treatment. Int Ophthalmol Clin. 2011; 51:135–144.
41. Novak KD, Williams SM, Kokot I, Schultze RL. Nd:YAG photodestruction of a presumed corneal Toxocara canis larva. Cornea. 2010; 29:703–705.

42. Lyall DA, Hutchison BM, Gaskell A, Varikkara M. Intravitreal Ranibizumab in the treatment of choroidal neovascularisation secondary to ocular toxocariasis in a 13-year-old boy. Eye (Lond). 2010; 24:1730–1731.

43. Hook EW 3rd, Peeling RW. Syphilis control--a continuing challenge. N Engl J Med. 2004; 351:122–124.
44. Fonollosa A, Giralt J, Pelegrin L, Sanchez-Dalmau B, Segura A, Garcia-Arumi J, et al. Ocular syphilis--back again: understanding recent increases in the incidence of ocular syphilitic disease. Ocul Immunol Inflamm. 2009; 17:207–212.

45. Kwak HD, Kim HW, Lee JE, Lee JE, Lee SJ, Yun IH. Clinical manifestations of syphilitic uveitis in the Korean population. J Korean Ophthalmol Soc. 2014; 55:555–562.

46. Pichi F, Ciardella AP, Cunningham ET Jr., Morara M, Veronese C, Jumper JM, et al. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina. 2014; 34:373–384.

48. Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010; 59:1–110.
49. Julie H, aNAR T. Spirochetal Infections. In : Ryan SJ, editor. Retina. 5th ed. 2013.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download