Abstract
Thyroid ophthalmopathy (TO) is an autoimmune inflammatory disorder involving the orbit characterized by inflammation and swelling of the extraocular muscles and an increase in orbital fat and connective tissue. Despite extensive research, TO continues to be a difficult condition for the patient to cope with and for the clinician to treat. Current treatments consist of systemic immunosuppression, orbital irradiation, and surgery. It is promising for patient refractory to conventional therapy that pathogenesis of TO at molecular level which advance development of new therapies targeting cellular immunity are now better understood. Future therapies targeting immune system or specific molecules are under investigation and show promise for the future. This review will describe current trends in the management of TO, from well-established therapies such as glucocorticoids, orbital irradiation and orbital decompression to more innovative therapies targeting immune system or specific molecules involved in TO pathogenesis.
Thyroid ophthalmopathy (TO), also known as thyroid eye disease or Graves' orbitopathy, is an autoimmune inflammatory disorder involving the orbit. Ninety percent of patients with TO are hyperthyroid, 5% are hypothyroid and another 5% are euthyroid [1]. In a few patients, TO precedes hyperthyroidism [2345]. Rather than being a complication of Graves' disease, TO seems to be a concomitant expression of the same underlying pathological autoimmune process directed against cross-reactive autoantigens in the thyroid and retrobulbar tissues [6789].
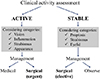
In newly diagnosed Graves' disease, 20% have mild and inactive TO, 5.8% present with moderate to severe, active TO and 0.3% develop compressive optic neuropathy [10]. The natural course of TO is typically characterized by an active phase followed by disease stabilization, as described by the "Rundle curve" [101112131415]. An early diagnosis of the active disease phase is important since immunosuppressive and disease-modifying therapies exert their effects only on active TO, while subjects with burn-out TO may only benefit from rehabilitative surgery [16]. The general algorithm for the management of TO is summarized in Fig. 1.
One goal of TO treatment is to control the modifiable risk factors. Uncontrolled hypothyroidism and hyperthyroidism, radioactive iodine therapy and smoking with dose-dependent manner have been associated with development of TO [1718192021222324]. Cessation of smoking, achievement of euthyroidism and prophylactic oral prednisolone prior to radioactive iodine therapy in at-risk patients form important preventive steps to control these modifiable risk factors for TO [242526].
Although severe TO is rare, it can cause irreversible loss of vision related to corneal exposure and compressive optic neuropathy, and such patients should be urgently treated to avoid devastating sight loss. Despite the management of TO is improved over the decades, an efficient therapeutic strategy remains still elusive, as most patients experience deterioration of their quality of life. This review will describe current trends in the management of TO, from well-established therapies such as glucocorticoids, orbital irradiation and orbital decompression to more innovative treatments targeting immune system or specific molecules involved in TO pathogenesis.
To date, corticosteroids are the treatment of choice for moderate to severe active TO. Numerous different oral and intravenous regimes are reported, with no clear consensus on the optimum dosage, dosing intervals, and duration of treatment that is undergoing continued refinement [27]. In 2005, Kahaly et al. [28] reported a cohort of 70 consecutive patients with severe active TO randomized to receive either intravenous methyl prednisolone (500 mg methylprednisolone once weekly for 6 weeks, then 250 mg once weekly for 6 weeks) or oral prednisolone (100 mg daily for a week with a gradual taper over 3 months). A positive clinical response was observed in 77% for the intravenous group compared with 51% of those receiving oral treatment. Subsequent studies have shown that pulsed intravenous methyl prednisolone is more effective [2930313233] and has a better safety compared with oral prednisone [283435363738]. More recently, a retrospective study with 50 patients reported that combination therapy can be effective in reducing clinical activity score (CAS) values as well as a low rate of severe side effects [39]. In this study, patients received 500 mg of intravenous methylprednisolone and repeated after 48 hours and then followed by tapering doses of oral prednisone and the cycle repeated each month for the next 5 months [39].
For prevention of radioactive iodine (RAI) induced exacerbation of TO, oral corticosteroids is widely used in daily practice, and prednisone at a dose of 0.2 mg/kg per body weight started 1 day after RAI and withdrawn after 6 weeks can be used [40]. A meta-analysis that evaluated oral prednisone treatment in the setting of RAI therapy suggested that oral prednisone (0.4-0.5 mg/kg) should be used for patients with mild to moderate TO, whereas low-dose prednisone (0.2-0.3 mg/kg) should be used for mild TO and for those patients without pre-existing TO, but with risk factors for developing it [41].
The limitation of intravenous methylprednisolone treatment is that 20-30% of patients are poorly responsive or unresponsive at all and that approximately 10-20% of patients present with disease relapse after drug withdrawal [38]. More recently, a study on a retrospective series of patients has shown that responsive patients have inactivation of TO as early as 6-8 weeks from the beginning of intravenous methylprednisolone, or may be otherwise switched to other treatments, alone or in combination with steroids [42].
With regard to dose, it is recommended that the total cumulative dose of intravenous corticosteroid should not exceed 8 g in consideration of steroid-related adverse effects [36]. A recent prospective observation showed that a cumulative dose of less than 4.5 g had good tolerance and low morbidity [43]. However, in terms of treatment response, a recent multicenter double blind randomized study has shown that a cumulative dose of 7.5 g of methylprednisolone has a higher response rate in patients with moderate-severe TO, when compared with intermediate or lower doses (5 g or 2.25 g, respectively), although it is associated with more frequent adverse events [38].
The morbidity and mortality of corticosteroid therapy in TO patients have been estimated to 6.5 and 0.6%, respectively [36]. Corticosteroids treatment may be accompanied by adverse effects, including liver dysfunction, hypertension, peptic ulcer disease, diabetes, infection, psychosis, or glaucoma [28444546]. A marked increase of liver enzymes is the most common adverse event associated with intravenous methylprednisolone. In order to reduce complications, careful screening of risk factors is mandatory. Before the treatment, patients should be screened for hepatitis virus markers and autoantibodies related to autoimmune hepatitis, and clinical monitoring should be regularly performed in order to promptly identify and treat complications [36].
The mainstay treatments for immunosuppression have been systemic corticosteroids, but which has significant adverse effects mentioned above. Therefore, novel immunomodulating agents targeting several antigens including B cells, tumor necrosis factor (TNF), thyroid stimulating hormone receptor (TSHR), insulin like growth factor-1 (IGF-1) receptor, interleukin-6 (IL-6) receptor and various inflammatory cytokines has been studied with the purpose of modifying the natural course of disease and sparing corticosteroid [4748].
Rituximab, an anti-CD20 monoclonal antibody that targets CD20 on B cells and its precursors is most actively researched immunosuppressive agent [49]. A systematic review of 43 TO cases treated with rituximab showed improvement in disease activity and severity in 91% [49]. A randomized controlled trial in Europe comparing rituximab to intravenous methylprednisolone in active moderate-severe TO supports effectiveness and disease-modifying effects with 100% response rate, no reactivation of TO at 24 weeks and less rehabilitative surgery required at 76 weeks [50]. However, a prospective randomized, double-blinded, placebo-controlled trial comparing rituximab to placebo did not show a significant difference in the improvement of disease activity at 24 and 52 weeks, and there were more moderate-to-severe adverse events in the rituximab group [51]. As so, the effect of rituximab remain conflicting in the treatment of TO.
There are small number of researches about anti-TNF monoclonal antibodies adalimumab and soluble TNF receptor etanercept in patients with active TO [525354]. Adalimumab reduced inflammatory score in 6 of 10 patients, the greatest benefit being seen in active TO with severe inflammatory signs [54]. Etanercept was found to be effective in controlling activity of TO, leading marked improvement in mainly soft tissue changes reported at 60%, but up to 30% had recurrence of TO activity after treatment cessation [52].
A recently developed drug-like small molecule antagonist of TSHR binds to TSHR and blocks basal and stimulated signal transduction [55]. Because cAMP, pAkt, and hyaluronan production are fibroblast functions that are activated via TSHR signaling and are important in the pathogenesis of TO, small molecule TSHR antagonists may prove to be effective in the treatment or prevention of the disease in the future [55].
The IGF-1 receptor is coexpressed on orbital fibroblasts along with the TSH-R and in vitro blocking of the IGF-1R attenuates TSH-dependent signaling [56]. In recent study, it was found that fibrocytes treated with teprotumumab, a human monoclonal antibody that blocks the IGF-1 receptor were found to have lower levels of expression of IGF-1 receptor and TSH receptor, suggesting teprotumumab could have a role in reducing or even preventing TO [56].
Another important pathway in active TO is represented by the IL-6/soluble IL-6 receptor system. Elevated serum soluble IL-6R concentrations were in fact measured in patients with active TO [57]. Tocilizumab, a recombinant, humanised monoclonal antibody to IL-6 receptor, has been trialled in 18 patients with active TO refractory to intravenous steroids. After tocilizumab infusion, CAS improved in 100% patients, proptosis decreased in 72%, and ocular motility improved in 83%. In this cohort, one patient with compressive optic neuropathy improved, avoiding orbital decompression [58].
Orbital lymphocytes and fibroblasts are sensitive to ionizing radiation [59] which is particularly effective in improving muscle motility during active disease [60]. Although a typical radiotherapy dose is 20 Gy, given in 10 fractions of 2 Gy, lower doses may be equally as effective [61]. A cumulative doses between 10 and 20 Gy may be effective [61]. Orbital irradiation is best effective in patients in whom irradiation was given shortly after the onset of symptoms and relatively ineffective against fibrotic extraocular muscle restriction or strabismus and has no effect on chronic proptosis [62].
Orbital irradiation is found to be effective for tapering off corticosteroid [63] and more effective when a combination of oral glucocorticoids and orbital irradiation is used rather than either treatment alone [59]. Orbital irradiation combined with long-term azathioprine has been found to be effective in thyroid-related restrictive myopathy [64].
Orbital irradiation should be used with caution in diabetic patients due to the risk of new or deteriorating diabetic retinopathy and preferably restricted to patients older than 35 years of age due to the long latency of radiotherapy-induced tumors [65].
Orbital decompression is a surgical procedure in that bone and fat removal may be performed separately or combined to maximize decompression. The choice of surgery is dependent on a combination of individual surgeon's preference and intersubject variability in orbital morphology [6667]. Immediate decompression surgery has no advantage over intravenous steroid therapy for improvement of visual acuity in the short term and surgical manipulation may worsen orbital inflammation if performed during the active phase [68]. However, in compressive optic neuropathy, when a prompt and adequate response is not seen with high dose systemic steroid, surgical decompression should be considered. In compressive optic neuropathy, the goal of orbital decompression is to relieve the hydrostatic pressure at the orbital apex, therefore reduce orbital congestion and improve vascular perfusion and axonal flow within the optic nerve [69]. The retrocaruncular transconjunctival approach is useful for removing the bony medial wall as far posteriorly as the annulus of Zinn allowing wide visualization of the entire medial orbital wall for effective decompression of the orbital apex with no visible scar and minimal tissue injury [69]. For two wall decompression, the conjunctival incision can be extended into the inferomedial conjunctival fornix to allow adequate exposure to decompress the anterior orbital floor medial to the infra-orbital nerve [69]. In more severe cases, the full three wall including lateral wall can also be decompressed through a lower lid swinging flap alone [69]. The balanced orbital decompression including simultaneous medial and lateral wall decompressions is thought to reduce the risk of postoperative diplopia by 'balancing' the amount of decompression on both sides of the orbit, thus reducing horizontal globe shifts [70]. In patients with compressive optic neuropathy who fail three-wall decompressions, the addition of transcranial orbital roof decompression can be performed [71]. The previous report about transcranial approach, showed improved of visual acuity and visual field and reduced proptosis with no change in diplopia after 5 years of follow-up [71].
Diplopia after decompression surgery is not uncommon that is why orbital decompression is performed prior to strabismus surgery in the management of TO. A recent study reported that opening the periorbita was associated with an increased incidence of new-onset diplopia [72]. Opening the periorbita allows orbital soft-tissue content herniation, but may have an undesired effect of increased diplopia. In that report, when 123 patients undergoing medial wall and strut sparing floor decompression surgery were reviewed, in cases with periorbita opening, the incidence of new diplopia was 26.8% compared to 10.8% in cases in which periorbita remained intact [72].
As for the normalization of visual function after decompression surgery, almost half of patients have persistent visual field defects [73]. However, recently reported case series of patients with no-light perception vision from dysthyroid optic neuropathy with onset of 5 days to 3 months showed a return of vision following orbital decompression suggesting that axonal death may be delayed by months after total nerve function loss and that decompression may still be effective in reversing compressive optic neuropathy in patients with no-light perception vision of up to 3 months [74].
Despite extensive research, TO continues to be a difficult condition for the patient to cope with and for the clinician to treat. Patients with TO should be treated according to the severity of their eye disease, keeping in mind that TO has a broad spectrum of clinical manifestations and a highly variable natural course. Current treatments consist of systemic immunosuppression, orbital irradiation, and surgery. Along with these treatments, achieving euthyroidism and smoking cessation should be emphasized. It is promising for patient refractory to conventional therapy that pathogenesis of TO at molecular level which advance development of new therapies targeting cellular immunity are now better understood. Future therapies targeting immune system or specific molecules are under investigation and show promise for the future.
References
1. Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994; 92:477–588.
3. Khoo DH, Eng PH, Ho SC, Tai ES, Morgenthaler NG, Seah LL, et al. Graves' ophthalmopathy in the absence of elevated free thyroxine and triiodothyronine levels: Prevalence, natural history, and thyrotropin receptor antibody levels. Thyroid. 2000; 10:1093–1100.

4. Marcocci C, Bartalena L, Bogazzi F, Panicucci M, Pinchera A. Studies on the occurrence of ophthalmopathy in Graves-disease. Acta Endocrinologica. 1989; 120:473–478.
5. Melcescu E, Horton WB, Pitman KT, Vijayakumar V, Koch CA. Euthyroid Graves' orbitopathy and incidental papillary thyroid microcarcinoma. Hormones (Athens). 2013; 12:298–304.

7. Fernando R, Atkins S, Raychaudhuri N, Lu Y, Li B, Douglas RS, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci U S A. 2012; 109:7427–7432.

8. Gillespie EF, Papageorgiou KI, Fernando R, Raychaudhuri N, Cockerham KP, Charara LK, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012; 97:E740–E746.

9. Bahn RS. Mechanisms of disease Graves' ophthalmopathy. N Engl J Med. 2010; 362:726–738.
10. Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, et al. Prevalence and natural history of Graves' orbitopathy in a large series of patients with newly diagnosed graves' hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013; 98:1443–1449.

11. Menconi F, Profilo MA, Leo M, Sisti E, Altea MA, Rocchi R, et al. Spontaneous improvement of untreated mild graves' ophthalmopathy: rundle's curve revisited. Thyroid. 2014; 24:60–66.

12. Marcocci C, Marino M. Treatment of mild, moderate-to-severe and very severe Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012; 26:325–337.

13. Perros P, Crombie AL, Kendalltaylor P. Natural-history of thyroid-associated ophthalmopathy. Clin Endocrinol (Oxf). 1995; 42:45–50.
14. Streeten DHP, Anderson GH, Reed GF, Woo P. Prevalence, natural-history and surgical-treatment of exophthalmos. Clin Endocrinol (Oxf). 1987; 27:125–133.
15. Noth D, Gebauer M, Muller B, Burgi U, Diem P. Graves' ophthalmopathy: natural history and treatment outcomes. Swiss Med Wkly. 2001; 131:603–609.
17. Tallstedt L, Lundell G, Torring O, Wallin G, Ljunggren JG, Blomgren H, et al. Occurrence of ophthalmopathy after treatment for Graves hyperthyroidism. N Engl J Med. 1992; 326:1733–1738.

18. Prummel MF, Wiersinga WM, Mourits MP, Koornneef L, Berghout A, Van der gaag R. Effect of abnormal thyroid-function on the severity of Graves ophthalmopathy. Arch Intern Med. 1990; 150:1098–1101.

19. Kung AW, Yau CC, Cheng A. The incidence of ophthalmopathy after radioiodine therapy for Graves-disease - prognostic factors and the role of methimazole. J Clin Endocrinol Metab. 1994; 79:542–546.

20. Hagg E, Asplund K. Is endocrine ophthalmopathy related to smoking? Br Med J (Clin Res Ed). 1987; 295:634–635.

21. Shine B, Fells P, Edwards OM, Weetman AP. Association between Graves ophthalmopathy and smoking. Lancet. 1990; 335:1261–1263.

22. Tellez M, Cooper J, Edmonds C. Graves' ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocrinol (Oxf). 1992; 36:291–294.

23. Prummel MF, Wiersinga WM. Smoking and risk of Graves-disease. JAMA. 1993; 269:479–482.
24. Thornton J, Kelly SP, Harrison RA, Edwards R. Cigarette smoking and thyroid eye disease: a systematic review. Eye. 2007; 21:1135–1145.

25. Bartalena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell'Unto E, et al. Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med. 1998; 338:73–78.

26. Tallstedt L, Lundell G, Blomgren H, Bring J. Does early administration of thyroxine reduce the development of Graves ophthalmopathy after radioiodine treatment. Eur J Endocrinol. 1994; 130:494–497.

27. Wiersinga WM. Autoimmunity in Graves' ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab. 2011; 96:2386–2394.

28. Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in graves' orbitopathy. J Clin Endocrinol Metab. 2005; 90:5234–5240.

29. Menconi F, Marino M, Pinchera A, Rocchi R, Mazzi B, Nardi M, et al. Effects of total thyroid ablation versus near-total thyroidectomy alone on mild to moderate Graves' orbitopathy treated with intravenous glucocorticoids. J Clin Endocrinol Metab. 2007; 92:1653–1658.

30. Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the european group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid. 2008; 18:333–346.

31. Matejka G, Verges B, Vaillant G, Petit JM, Brun-Pacaud A, Rudoni S, et al. Intravenous methylprednisolone pulse therapy in the treatment of Graves' ophthalmopathy. Horm Metab Res. 1998; 30:93–98.

32. Kendall-Taylor P, Crombie AL, Stephenson AM, Hardwick M, Hall K. Intravenous methylprednisolone in the treatment of Graves ophthalmopathy. BMJ. 1988; 297:1574–1578.

33. Aktaran S, Akarsu E, Erbagci I, Araz M, Okumus S, Kartal M. Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves' ophthalmopathy. Int J Clin Pract. 2007; 61:45–51.

34. Kauppinen-Makelin R, Karma A, Leinonen E, Loyttyniemi E, Salonen O, Sane T, et al. High dose intravenous methylprednisolone pulse therapy versus oral prednisone for thyroid-associated ophthalmopathy. Acta Ophthalmol Scand. 2002; 80:316–321.

35. Macchia PE, Bagattini M, Lupoli G, Vitale M, Vitale G, Fenzi G. High dose intravenous corticosteroid therapy for Graves' ophthalmopathy. J Endocrinol Invest. 2001; 24:152–158.

36. Zang S, Ponto KA, Kahaly GJ. Intravenous glucocorticoids for Graves' orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011; 96:320–332.

37. Gao GH, Dai JH, Qian YF, Ma F. Meta-analysis of methylprednisolone pulse therapy for Graves' ophthalmopathy. Clin Exp Ophthalmol. 2014; 42:769–777.

38. Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves' orbitopathy. J Clin Endocrinol Metab. 2012; 97:4454–4463.

39. Nedeljkovic Beleslin B B, Ciric J, Zarkovic M, Stojkovic M, Savic S, Knezevic M, et al. Efficacy and safety of combined parenteral and oral steroid therapy in Graves' orbitopathy. Hormones (Athens). 2014; 13:222–228.

40. Lai A, Sassi L, Compri E, Marino F, Sivelli P, Piantanida E, et al. Lower dose prednisone prevents radioiodine-associated exacerbation of initially mild or absent Graves' orbitopathy: a retrospective cohort study. J Clin Endocrinol Metab. 2010; 95:1333–1337.

41. Shiber S, Stiebel-Kalish H, Shimon I, Grossman A, Robenshtok E. Glucocorticoid regimens for prevention of Graves' ophthalmopathy progression following radioiodine treatment: systematic review and meta-analysis. Thyroid. 2014; 24:1515–1523.

42. Vannucchi G, Covelli D, Campi I, Origo D, Curro N, Cirello V, et al. The therapeutic outcome to intravenous steroid therapy for active Graves' orbitopathy is influenced by the time of response but not polymorphisms of the glucocorticoid receptor. Eur J Endocrinol. 2014; 170:55–61.

43. Riedl M, Kolbe E, Kampmann E, Kramer I, Kahaly GJ. Prospectively recorded and MedDRA-coded safety data of intravenous methylprednisolone therapy in Graves' orbitopathy. J Endocrinol Invest. 2015; 38:177–182.

44. Marino M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C. Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves' ophthalmopathy. Thyroid. 2004; 14:403–406.

45. Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM. Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves' ophthalmopathy. Thyroid. 2007; 17:357–362.

46. Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell'Unto E, Rocchi R, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves' ophthalmopathy: Results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab. 2001; 86:3562–3567.

47. Salvi M. Immunotherapy for Graves' ophthalmopathy. Curr Opin Endocrinol Diabetes Obes. 2014; 21:409–414.

48. Bartalena L. Commentary: rituximab, adalimumab, etanercept, tocilizumab-are biologics the future for Graves' orbitopathy? Ophthal Plast Reconstr Surg. 2014; 30:420–423.
49. Salvi M, Vannucchi G, Beck-Peccoz P. Potential utility of rituximab for Graves' orbitopathy. J Clin Endocrinol Metab. 2013; 98:4291–4299.

50. Salvi M, Vannucchi G, Curro N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves' orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015; 100:422–431.

51. Stan MN, Garrity JA, Leon BGC, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves' orbitopathy. J Clin Endocrinol Metab. 2015; 100:432–441.

52. Paridaens D, van den Bosch WA, van der Loos TL, Krenning EP, van Hagen PM. The effect of etanercept on Graves' ophthalmopathy: a pilot study. Eye. 2005; 19:1286–1289.

53. Durrani OM, Reuser TQ, Murray PI. Infliximab: a novel treatment for sight-threatening thyroid associated ophthalmopathy. Orbit. 2005; 24:117–119.

54. Ayabe R, Rootman DB, Hwang CJ, Ben-Artzi A, Goldberg R. Adalimumab as steroid-sparing treatment of inflammatory-stage thyroid eye disease. Ophthal Plast Reconstr Surg. 2014; 30:415–419.

55. Turcu AF, Kumar S, Neumann S, Coenen M, Iyer S, Chiriboga P, et al. A small molecule antagonist inhibits thyrotropin receptor antibody-induced orbital fibroblast functions involved in the pathogenesis of Graves ophthalmopathy. J Clin Endocrinol Metab. 2013; 98:2153–2159.

56. Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, et al. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. 2014; 99:E1635–E1640.

57. Slowik M, Urbaniak-Kujda D, Bohdanowicz-Pawlak A, Kapelko-Slowik K, Dybko J, Wolowiec D, et al. CD8+CD28-lymphocytes in peripheral blood and serum concentrations of soluble interleukin 6 receptor are increased in patients with Graves' orbitopathy and correlate with disease activity. Endocr Res. 2012; 37:89–95.

58. Perez-Moreiras JV, Alvarez-Lopez A, Gomez EC. Treatment of active corticosteroid-resistant Graves' orbitopathy. Ophthal Plast Reconstr Surg. 2014; 30:162–167.

59. Bartalena L, Pinchera A, Marcocci C. Management of Graves' ophthalmopathy: reality and perspectives. Endocr Rev. 2000; 21:168–199.

60. Prummel MF, Terwee CB, Gerding MN, Baldeschi L, Mourits MP, Blank L, et al. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves' ophthalmopathy. J Clin Endocrinol Metab. 2004; 89:15–20.

61. Kahaly GJ, Rosler HP, Pitz S, Hommel G. Low- versus high-dose radiotherapy for Graves' ophthalmopathy: A randomized, single blind trial. J Clin Endocrinol Metab. 2000; 85:102–108.

62. Melcescu E, Horton WB, Kim D, Vijayakumar V, Corbett JJ, Crowder KW, et al. Graves orbitopathy: update on diagnosis and therapy. South Med J. 2014; 107:34–43.

63. Hahn E, Laperriere N, Millar BA, Oestreicher J, McGowan H, Krema H, et al. Orbital radiation therapy for Graves' ophthalmopathy: measuring clinical efficacy and impact. Pract Radiat Oncol. 2014; 4:233–239.

64. Chalvatzis NT, Tzamalis AK, Kalantzis GK, El-Hindy N, Dimitrakos SA, Potts MJ. Safety and efficacy of combined immunosuppression and orbital radiotherapy in thyroid-related restrictive myopathy: two-center experience. Eur J Ophthalmol. 2014; 24:953–959.

65. Marcocci C, Bartalena L, Rocchi R, Marino M, Menconi F, Morabito E, et al. Long-term safety of orbital radiotherapy for Graves' ophthalmopathy. J Clin Endocrinol Metab. 2003; 88:3561–3566.

66. Wu CH, Chang TC, Liao SL. Results and predictability of fat-removal orbital decompression for disfiguring graves exophthalmos in an Asian patient population. Am J Ophthalmol. 2008; 145:755–759.

67. Borumandi F, Hammer B, Kamer L, von Arx G. How predictable is exophthalmos reduction in Graves' orbitopathy? A review of the literature. Br J Ophthalmol. 2011; 95:1625–1630.

68. Wakelkamp IM, Baldeschi L, Saeed P, Mourits MP, Prummel MF, Wiersinga WM. Surgical or medical decompression as a first-line treatment of optic neuropathy in Graves' ophthalmopathy? A randomized controlled trial. Clin Endocrinol (Oxf). 2005; 63:323–328.

69. Verity DH, Rose GE. Acute thyroid eye disease (TED): principles of medical and surgical management. Eye. 2013; 27:308–319.

70. Rao R, MacIntosh PW, Yoon MK, Lefebvre DR. Current trends in the management of thyroid eye disease. Curr Opin Ophthalmol. 2015; 26:484–490.

71. Bingham CM, Harris MA, Vidor IA, Rosen CL, Linberg JV, Marentette LJ, et al. Transcranial orbital decompression for progressive compressive optic neuropathy after 3-wall decompression in severe Graves' orbitopathy. Ophthal Plast Reconstr Surg. 2014; 30:215–218.

72. Mainville NP, Jordan DR. Effect of orbital decompression on diplopia in thyroid-related orbitopathy. Ophthal Plast Reconstr Surg. 2014; 30:137–140.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download