INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic, autoimmune, connective tissue disorder of multiple organ systems (joints, skin, kidneys, and brain, among others) affecting about 20-150 patients per 100,000 people. It typically has a relapsing and remitting clinical course. The eye is frequently involved in SLE [1], and the disease may cause ocular involvement by several mechanisms including immune complex deposition in the basement membrane of small blood vessel endothelial cells [1]. Ocular complications have been reported in up to one-third of patients with SLE [2]. Ocular manifestations can be associated with significant morbidity and may play a role as a marker for systemic disease activity. The onset of disease is usually between the late teens and early 40s and women have a nine-fold higher incidence of the disease in comparison to men [34]. SLE can affect various parts of the eye from the orbit and external eye to the retina and optic nerve [25]. This review will show the various ocular manifestations of SLE and discuss treatment options for eye problems.
PATHOPHYSIOLOGY
The pathogenesis of SLE is complex and incompletely understood. Environmental factors and hormonal influences, among other factors, may be involved in the development of lupus. SLE is associated with defects in the innate and adaptive immune systems, apoptotic clearance, cytokines, T-cell signaling and B-cell immunity [6]. Ischemic retinal vasculitis with vascular occlusion is a serious complication resulting in severe visual loss and late complications including neovascular glaucoma [7]. The exact mechanism of vascular occlusion remains unclear; however, some suggested possible pathogenic mechanisms include immune-complex deposition, complement activation with microvascular thrombosis and fibrinoid degeneration of the vascular wall [8]. Many elements of the immune system are involved in the pathogenesis of lupus, and autoantibodies appear to play a key role. Cellular death by apoptosis may expose self-antigens that are normally sequestered inside cells to antigen presenting cells (APCs), thus initiating a harmful immune response. T and B lymphocytes are then activated via various cellular interactions leading to the production of high-affinity autoantibodies. APCs release cytokines and other inflammatory factors that contribute to the immune reaction [91011]. As such, many treatments have been developed targeting T cells and B cells.
DIAGNOSIS
The clinical diagnosis of SLE is based on the presence of four of the 11 features listed by the American College of Rheumatology classification criteria [1213]. The presence of four criteria indicates a diagnosis of SLE, serially or simultaneously, during the course of the disease. The revised criteria include: (1) malar rash, (2) discoid rash, (3) skin photosensitivity, (4) oral ulcers, (5) nonerosive arthritis, (6) serositis, (7) renal involvement, (8) neurological disorder, (9) hematologic disorder, (10) immunologic disorder, and (11) positive antinuclear antibodies. The presence of 4 of these 11 criteria confirms the diagnosis of SLE and yields a sensitivity of 85% and a specificity of 95% for SLE [14].
OCULAR MANISFESTATIONS
One-third of SLE patients have ophthalmic involvement and the most common ocular problem is keratoconjunctivitis sicca. SLE can affect various ocular structures including the cornea, conjunctiva, episclera, sclera, retina, uveal tract, optic nerve, vasculature, orbit, and adnexa. A summary of SLE ocular manifestations is given in Table 1. The prevalence and visual prognosis differ among each involved structure.
1. External ocular structures
1) Orbit and eyelid
Orbital involvement is rare in SLE. Most orbital problems develop bilaterally. Orbital inflammation including myositis and panniculitis has been reported [1516]. Orbital inflammation and vasculitis result in vision loss due to ischemic injury in the optic nerve and increased intraocular pressure due to neovascular glaucoma [2]. Orbital myositis is accompanied by pain, globe restriction, periorbital swelling and proptosis [17]. Subcutaneous orbital involvement and periocular skin inflammation is rarely developed. Discoid lupus erythematosus is associated with raised, scaly and atrophic skin lesions. Sometimes these eyelid lesions can easily be misdiagnosed as simple blepharitis and/or eczema [25] Lid biopsy and direct immunohistochemistry studies can be used for diagnosis confirmation.
2) Lacrimal system
Keratoconjunctivitis sicca is the most common ocular manifestation of SLE. Clinical indications include eye discomfort, exposure keratopathy, and symblepharon formation [218] and the disease is often related to secondary Sjögren's syndrome (SS) [5]. The International Dry Eye WorkShop classified Sjögren's as an aqueous tear-deficient dry eye, reflecting failure of lacrimal tear secretion. The Schirmer I test (≤5 mm in 5 min) (Fig. 1) or rose Bengal score (≥4 according to the van Bijsterveld scoring system) are important tests for the diagnosis of dry eye syndrome-associated SS [19]. Proinflammatory markers can be detected in the tear film of SLE patients. Corneal opacity, ulceration, and recurrent keratitis are possible complications of severe dry eye that can occur to threaten visual acuity [5].
2. Anterior segment diseases
1) Corneal diseases
Corneal involvement in SLE patients has a wide spectrum of range of severity, which may present as very mild superficial punctate keratitis or ulcerative keratitis. Superficial punctate keratitis commonly develops secondary to SS.
2) Episclera and sclera
Episcleritis is a painless, mildly uncomfortable red eye with episcleral vessel dilation that is markedly decreased by treatment with topical phenylephrine. On the other hand, scleritis is a severe vision-threatening destructive inflammatory condition. Both of these conditions are often associated with systemic inflammatory diseases. According to a large cohort study, 35.8% of scleritis patients and 27.1% of episcleritis patients have underlying systemic disease [20].
3. Posterior segment diseases
1) Lupus retinopathy: microangiopathy and severe vaso-occlusive retinopathy
Lupus retinopathy is a potentially vision-threatening ocular manifestation of SLE that occurs in about half of lupus patients. However, with steroids and immunosuppressive therapy, the incidence of lupus retinopathy has decreased remarkably. The prevalence of lupus retinopathy tends to increase along with systemic disease activity. Retinal involvement is associated with systemic activity and CNS lupus. Retinopathy is first evidenced by small intraretinal hemorrhages and cotton wool spots. Most patients with lupus retinopathy have a mild, nonischemic form; however, vascular occlusion can be visualized via fundus fluorescein angiography, and manifests as widespread arteriolar or branch retinal artery occlusion accompanied by retinal ischemia and neovascularization in some patients with ischemic-type lupus retinopathy.
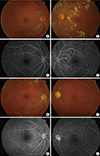
Severe vaso-occlusive retinopathy is a rare but well-described entity that is associated with extensive capillary nonperfusion, multiple branch retinal artery and vein occlusions resulting in ocular neovascularization, vitreous hemorrhage, tractional retinal detachment and neovascular glaucoma (Fig. 2). Retinal vasculitis is associated with poor visual outcomes, an acute presentation and it can be the first manifestation of SLE. In many cases of vaso-occlusive retinopathy, retinal vasculitis is accompanied by antiphospholipid antibodies [2223]. One half of eyes with severe vaso-occlusive retinopathy might suffer vision loss worse than 20/200 [24].
2) Lupus choroidopathy
Other rare retinal manifestations of lupus include chorioidopathy with serous retinal detachments similar to funduscopic findings of central serous chorioretinopathy
Corticosteroid-induced central serous chorioretinopathy may develop that makes it challenging to treat serous retinal detachment. For the appropriate treatment of serous retinal detachment in patients with SLE, it is very important to differentiate lupus choroidopathy from steroid-induced central serous chorioretinopathy [25].
4. Drug-induced ophthalmic complications
The most common ocular adverse events of SLE treatment are related to the use of corticosteroids. Steroid-induced ocular problems include cataract formation, steroid-induced glaucoma, and steroid-induced central serous chorioretinopathy [21].
TREATMENT
SLE treatment varies depending on the organs involved and the severity of disease. Because of the systemic nature of the disease involving multiple organs, collaboration among the involved specialists (ophthalmologists, rheumatologists, nephrologists, dermatologists) is often required for tailored therapy for each patient.
Traditional treatment options for SLE include non-steroidal anti-inflammatory drugs, corticosteroids, anti-malarial drugs and other immunosuppressive agents. Corticosteroids are the mainstay acute treatment for treating ocular SLE. Corticosteroids are fast acting and effective in short-term use; however, corticosteroid-sparing agents should be considered for long-term therapy [26].
For patients with mild disease, NSAIDs and antimalarials are chosen for treatment [422]. Antimalarials, such as chloroquine and hydroxychloroquine, have been reported to be effective in treating SLE; however, the antimalarial drugs are associated with dose-related retinal toxicity. Retinal toxicity remains a concern, with ongoing debate with regard to how best to monitor patients taking antimalarials.
In 2011 the American Academy of Ophthalmology (AAO) suggested the recommendations for chloroquine and hydroxychloroquine screening. The AAO recommends a baseline examination within the first year of use and annual screening for 5 years of use [27].
Plasmapheresis can be performed to eliminate circulating immune complexes and autoantibodies [28]. A variety of systemic immunosuppressants have demonstrated efficacy in treating ocular SLE. These therapies include methotrexate, mycophenolate mofetil, cyclosporine A, azathioprine, chlorambucil and cyclophosphamide [2930]. High-dose intravenous (IV) cyclophosphamide has been known to play a role in the treatment of SLE, especially in severe cases such as lupus nephritis, central nervous system lupus and vasculitis [31]. Some patients revealed refractory features [32]. In addition, generalized immunosuppression can result in serious side-effects including bone marrow suppression, hepatotoxicity and risk of infection. Biologics SLE involves production of multiple autoantibodies, each of which has been targeted as a potential immunotherapy. Thus, recently several biologic agents, targeting specific components of the immune system, are being evaluated for the treatment of SLE.
New biologic agents are playing an important role in the management of SLE (Table 2).
Because lupus is a multi-organ disease, an important step for the treatment is the assessment of disease activity across multiple systems. The SLE Disease Activity Index and British Isles Lupus Assessment Group (BILAG) index are the most widely used diagnosis systems [33]. Although systemic medication is required to treat the underlying disease, the ocular manifestations of SLE require additional local therapy in some cases. For example, keratoconjunctivitis sicca, the most common ocular manifestation of SLE, can be treated with artificial tears, punctal plugs and topical cyclosporine. Laser photocoagulation has been applied for the treatment of vaso-occlusive lupus retinopathy. Vitrectomy can also be performed in proliferative retinopathy with vitreous hemorrhage or tractional retinal detachment resulting from ocular ischemia [34]. In addition, anti-vascular endothelial growth factor has recently proven to be effective in the treatment of ischemic lupus retinopathy [2323]. Topical corticosteroids may be adequate for the treatment of anterior uveitis, or corneal ocular manifestations associated with SLE [4]. The presence of scleral, orbital, retinal, choroidal or neurological manifestations requires systemic therapy [5].
CONCLUSION
SLE is a potentially life-threatening multisystem disease that is commonly associated with ocular manifestations. This paper reviews recent advances in our understanding of the pathogenesis, diagnosis, and treatment of SLE. A better understanding of SLE pathophysiology will facilitate development of more effective treatment options. Although many new biologic treatments have been evaluated in the past decade, clear guidelines for treating SLE have not been established. A high clinical suspicion for lupus should be maintained for early diagnosis. Treatment of ophthalmic involvement should be selected via collaboration with a lupus specialist. More research is needed in order to determine which therapy and/or combination of therapies will provide the best outcome for SLE patients.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download