Abstract
Systemic hypertension affects not only the heart, kidneys, brain, and large arteries but also the eyes. High blood pressure (BP) causes a series of pathophysiological changes in the retinal vasculature, including focal and diffuse narrowing of the retinal arteriole, opacification of the arteriolar wall, and compression of the venules by arterioles. In severe cases, hemorrhage, nerve fiber layer infraction, and disc swelling can occur. Systemic hypertension results in various retinal vascular diseases, such as hypertensive retinopathy, retinal vein or artery occlusion, retinal arterial macroaneurysm, and nonarteritic ischemic optic neuropathy. High BP also increases the risk of development and progression of diabetic retinopathy. Signs of hypertensive retinopathy are predictive of target-organ damages, including cardiovascular and cerebrovascular diseases. While managing patients with hypertensive retinopathy, physicians should be aware of the management of cardiovascular and cerebrovascular risk factors.
High blood pressure (BP) affects not only the heart, kidneys, brain, and large arteries but also the eyes. Retinal, choroidal, and optic nerve circulations undergo pathophysiological changes, resulting in clinical signs referred to as hypertensive retinopathy, hypertensive choroidopathy, and hypertensive optic neuropathy. Systemic hypertension also increases the risk of development of retinal vein and artery occlusion, retinal-arteriolar emboli, retinal arterial macroaneurysm, nonarteritic ischemic optic neuropathy (NAION), and diabetic retinopathy.
On the other hand, the eye is the only organ where the blood vessels can be observed directly. Retinal arterioles are similar to the cardiac and cerebral arterioles from the anatomical and physiological standpoint. The evaluation of retinal circulation provides further information of the changes in the microvasculature in the body, which may provide additional information on the cardiovascular or cerebrovascular risk stratification of hypertensive patients [123].
The purpose of this review was to summarize the ocular manifestations of systemic hypertension and its clinical implication on cardiovascular and cerebrovascular risk stratification.
High BP results in a series of pathophysiological changes in the retinal circulation [4]. The initial response is vasospasm as well as an increase in the vasomotor tone (generalized arteriolar narrowing, Fig. 1). Chronic arteriosclerotic changes then develop, such as intimal thickening, media-wall hyperplasia, and hyaline degeneration. These changes are clinically seen as focal and diffuse narrowing of the retinal arterioles, opacification of the arteriolar wall (silver or copper wiring), and compression of the venules by arterioles at their common adventitial sheath (arteriovenous nipping or nicking, Fig. 1) (arteriosclerotic stage). At a much higher BP, the blood-retinal barrier breaks down, resulting in lipid exudation (hard exudates), hemorrhages, and nerve fiber layer infraction (cotton-wool spots, Fig. 1) (exudative stage). In patients with severe high BP, disc swelling (papilledema) can occur owing to optic nerve ischemia and concomitantly increased intracranial pressure (malignant stage). However, the above stages of hypertensive retinopathy are not always sequential.
Patients with systemic hypertension tend to also have diabetes or atherosclerosis. Approximately one-third of the individuals have increased BP when type 2 diabetes is first diagnosed [5], with the prevalence of systemic hypertension increasing to approximately 100% when diabetic renal disease manifests [6]. The concomitant presence of systemic hypertension, diabetes, and atherosclerosis significantly magnifies the risk and aggravates microvascular complications, including hypertensive or diabetic retinopathy [7].
Hypertensive retinopathy is a spectrum of retinal microvascular changes that are associated with high BP [8]. Keith et al. suggest that the severity of retinal microvascular changes predict the mortality of hypertensive patients and devised a four-grade classification system for hypertensive retinopathy, with increasing severity based on arterial characteristics and retinopathy ("Keith-Wagener-Baker system", Table 1) [9]. This is the most commonly used grade scale of retinal vasculature. The non-malignant signs (arteriovenous ratio and general and focal vessel narrowing) are graded compared to what the examiner considers to be normal. Additional malignant signs of hemorrhages, exudates, and papilledema are graded according to their presence or absence. The 3-year survival rate was 70% for patients with grade 1 hypertensive retinopathy compared to 6% for patients with grade 4 retinopathy [8].
Although this system is widely used, early retinopathy grades are not easily distinguished (e.g., grade 1 signs and grade 2 signs are difficult to distinguish). Furthermore, the prognostic implications of early hypertensive retinopathy grades are unclear. Thus, attempts have been made to grade the state based on the relative severity of hypertensive retinopathy signs, relation to systemic disease, and the prognostic significance [1]. In this system, mild retinopathy can be identified by retinal microvascular changes, including generalized and focal arteriolar narrowing, opacification of the arteriolar wall, and arteriovenous nipping. In addition to these signs, moderate retinopathy can be identified by blot- or flame-shaped hemorrhages, hard exudates, microaneurysms, cotton-wool spots, or a combination of all these signs. Severe retinopathy can show some or all of these retinopathy signs as well as optic disc swelling.
A recent study reported fundus findings in Korean hypertensive patients [10]. A total of 519 patients with systemic hypertension were evaluated and classified into hypertensive retinopathy grades I-IV, based on the grading scale proposed by Keith et al. [9]. Of the 308 patients with hypertensive retinopathy, 226 (73.4%) showed grade I hypertensive retinopathy; 71 (23.1%), grade II; 7 (2.2%), grade III; and 4 (1.3%), grade IV.
Retinal vein occlusion is the most common retinal vascular disorder, second to diabetic retinopathy, in the elderly population and is characterized by dilated and tortuous retinal veins, retinal hemorrhages, cotton-wool spots, and edema of the macula and optic disc [11]. These signs can be seen either in only one (branch retinal vein occlusion) or in all four quadrants (central retinal vein occlusion, Fig. 2). There is a strong association between systemic hypertension and retinal vein occlusion, especially in the older age group (over 50 years) as uncontrolled or recently diagnosed systemic hypertension is common among them [121314].
Retinal emboli are oval or rhomboid-shaped discrete plaque-like lesions lodged in the lumen of retinal arterioles (Fig. 3A) [15]. They can be single or multiple and can be seen in one or both the eyes [16]. The major risk factors for retinal emboli are known to be systemic hypertension, diabetes, and cigarette smoking [171819]. Retinal emboli can occlude the distal portions, resulting in retinal artery occlusion (Fig. 3B). Retinal artery occlusion is a sight-threatening condition characterized by a sudden, painless loss of vision with a whitened retina. Occlusion of the central retinal artery (central retinal artery occlusion) is more common than sectoral occlusion (branch retinal artery occlusion), resulting in retinal whitening of the posterior pole and a cherry-red spot [20].
Retinal arterial macroaneurysm is a saccular fusiform dilatation of a retinal arteriole and is associated with systemic hypertension in up to 75% of patients and results from dilatation of inelastic aging retinal arterioles due to high BP [21]. Patients are usually asymptomatic, but may present hemorrhaging and exudation, resulting in vision loss.
NAION is the most common acute optic nerve disease in elderly individuals. It presents with a sudden visual loss with optic-disc edema, which is typically absent in posterior optic nerve ischemia, and lacks evidence of temporal arteritis [22]. Systemic hypertension may impair the optic nerve perfusion in a similar way to that seen in retinal circulation. Up to 50% of the patients with NAION may have systemic hypertension [23].
Systemic hypertension is a risk factor for the development of diabetic retinopathy as well as its progression. In poorly controlled diabetic patients, retinal blood flow is commonly increased and the retinal vascular autoregulation is usually damaged. Moreover, high BP causes shear stress of the vessel walls that results in retinal vascular endothelial damage, followed by increased expression of vascular endothelial growth factors [2425]. Tight BP control in diabetic patients is important for both primary and secondary prevention of diabetic retinopathy [26].
The association of hypertensive retinopathy and the risk of coronary heart disease have been documented in previous reports. In the National Health Examination Survey, individuals with retinal arteriolar narrowing were two to six times more likely to have a preexisting coronary heart disease than those without retinal arteriolar narrowing, after adjusting the systemic hypertension, diabetes, and serum cholesterol levels [27]. Duncan et al. reported that the presence of hypertensive retinopathy predicted a doubling in the risk of coronary heart disease, and the presence of either generalized or focal narrowing of arterioles predicted almost a tripling of the risk in men with systemic hypertension and hyperlipidemia [28]. However, the Atherosclerosis Risk in Communities study reported that generalized arteriolar narrowing increases the risk of coronary heart disease only in women but not in men [21]. People with retinal emboli show a higher risk of cardiovascular disease [19] and are two times more likely to have coronary heart disease and four times more likely to have carotid artery plaque than those without emboli [29].
Kang et al. [10] evaluated the fundus findings and their association with target organ damages in Korean hypertensive patients. The respective proportions of patients with cardiovascular disease, including coronary artery diseases and left ventricular hypertrophy, in each grade of hypertensive retinopathy were 45.6% in grade I, 80.3% in grade II, 42.9% in grade III, and 50.0% in grade IV, but 37.4% in the non-hypertensive retinopathy group.
The evaluation of hypertensive retinopathy can be useful for the risk stratification of stroke. The Atherosclerosis Risk in Communities study showed that signs of retinopathy predicted a two to four times higher risk of newly diagnosed clinical stroke compared to those without signs after adjusting the other risk factors of stroke [30]. Likewise, population-based studies conducted in the United States [31] and in Japan [32] showed that the risk of stroke are two to three times higher in persons with signs of retinopathy and these associations were independent of cardiovascular risk factors. The presence of retinal emboli resulted in a two-fold higher risk of stroke mortality, which is independent of BP and other risk factors [18].
In the study of Kang et al., cerebrovascular disease was observed in 1.9% of persons with hypertensive retinopathy and was not observed in persons without hypertensive retinopathy [10].
Systemic hypertension is associated with the development of retinal vascular diseases, including hypertensive retinopathy, retinal vein or artery occlusion, retinal-arteriolar emboli, retinal arterial macroaneurysm, and NAION. Systemic hypertension also increases the risk of development and progression of diabetic retinopathy. Retinal vasculature changes are predictive and have prognostic value in target-organ damages of systemic hypertension, including cardiovascular and cerebrovascular diseases. Close monitoring and strict management of cardiovascular and cerebrovascular risk factors are recommended for the management of hypertensive patients with signs of retinopathy.
Figures and Tables
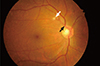 | Fig. 1Hypertensive retinopathy. A cotton wool spot (white arrow) and a compressed venule by an arteriole (black arrow). |
References
2. Liew G, Campbell S, Klein R, Klein BE, Sharrett AR, Cotch MF, et al. Ten-year longitudinal changes in retinal microvascular lesions: the atherosclerosis risk in communities study. Ophthalmology. 2011; 118:1612–1618.

3. Wang JJ, Liew G, Wong TY, Smith W, Klein R, Leeder S, et al. Retinal vascular calibre and the risk of coronary heart disease-related death. Heart. 2006; 92:1583–1587.

4. Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982; 89:1132–1145.

6. Tarnow L, Rossing P, Gall MA, Nielsen FS, Parving HH. Prevalence of arterial hypertension in diabetic patients before and after the JNC-V. Diabetes Care. 1994; 17:1247–1251.

7. Zoungas S, de Galan B, Ninomiya T, Grobbee D, Hamet P, Heller S, et al. ADVANCE Collaborative Group Cass A. Glasziou P, Harrap S, Lisheng L, Mancia G, Pillai A, Poulter N, Perkovic V, Travert F. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diabetes Care. 2009; 32:2068–2074.

8. Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001; 46:59–80.

9. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1974; 268:336–345.

10. Kang S, Roh YJ, Moon JI. Hypertensive retinopathy and associated target organ damage in Korean hypertensive patients. J Korean Ophthalmol Soc. 2010; 51:1231–1236.

11. David R, Zangwill L, Badarna M, Yassur Y. Epidemiology of retinal vein occlusion and its association with glaucoma and increased intraocular pressure. Ophthalmologica. 1988; 197:69–74.

12. Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000; 98:133–143.
13. Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, Kowalski JW, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1094–1101.e5.

14. McIntosh RL, Rogers SL, Lim L, Cheung N, Wang JJ, Mitchell P, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010; 117:1113–1123.e15.

15. Hollenhorst RW. Significance of bright plaques in the retinal arterioles. JAMA. 1961; 178:23–29.

16. Wong TY, Klein R. Retinal arteriolar emboli: epidemiology and risk of stroke. Curr Opin Ophthalmol. 2002; 13:142–146.

17. Mitchell P, Wang JJ, Li W, Leeder SR, Smith W. Prevalence of asymptomatic retinal emboli in an Australian urban community. Stroke. 1997; 28:63–66.

18. Klein R, Klein BE, Jensen SC, Moss SE, Meuer SM. Retinal emboli and stroke: The beaver dam eye study. Arch Ophthalmol. 1999; 117:1063–1068.
19. Klein R, Klein BE, Moss SE, Meuer SM. Retinal emboli and cardiovascular disease: the Beaver Dam Eye Study. Arch Ophthalmol. 2003; 121:1446–1451.
20. Marcucci R, Sodi A, Giambene B, Liotta AA, Poli D, Mannini L, et al. Cardiovascular and thrombophilic risk factors in patients with retinal artery occlusion. Blood Coagul Fibrinolysis. 2007; 18:321–326.

21. Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women: the Atherosclerosis Risk in Communities Study. JAMA. 2002; 287:1153–1159.
22. Hattenhauer MG, Leavitt JA, Hodge DO, Grill R, Gray DT. Incidence of Nonarteritic Anteripr Ischemic Optic Neuropathy. Am J Ophthalmol. 1997; 123:103–107.

23. Group IONDTS. Characteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the Ischemic Optic Neuropathy Decompression Trial. Arch Ophthalmol. 1996; 114:1366–1374.
24. Hsueh WA, Anderson PW. Hypertension, the endothelial cell, and the vascular complications of diabetes mellitus. Hypertension. 1992; 20:253–263.

25. Suzuma I, Hata Y, Clermont A, Pokras F, Rook SL, Suzuma K, et al. Cyclic Stretch and Hypertension Induce Retinal Expression of Vascular Endothelial Growth Factor and Vascular Endothelial Growth Factor Receptor—2 Potential Mechanisms for Exacerbation of Diabetic Retinopathy by Hypertension. Diabetes. 2001; 50:444–454.

26. Matthews DR. Group UPDS. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004; 122:1631–1640.

27. Gillum RF. Retinal arteriolar findings and coronary heart disease. Am Heart J. 1991; 122:262–263.

28. Duncan B, Wong T, Tyroler H, Davis C, Fuchs F. Hypertensive retinopathy and incident coronary heart disease in high risk men. Br J Ophthalmol. 2002; 86:1002–1006.

29. Wong TY, Larsen EKM, Klein R, Mitchell P, Couper DJ, Klein BE, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology. 2005; 112:540–547.

30. Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001; 358:1134–1140.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download