Abstract
Noise-induced hearing loss (NIHL) is the second most common cause of permanent hearing impairment after age-related hearing loss. NIHL is influenced by environmental and genetic factors and the effects of noise can be exacerbated by the administration of ototoxic drugs or exposure to chemicals. The pathophysiology of NIHL is classified as either mechanical injury or metabolic (or biochemical) injury. Exposure of cochleae to intense sounds has been found to disrupt the stereocilia on the hair cells by separating the tip links and to depolymerize actin filaments, resulting in a disturbance in signal transduction. Major mechanisms of metabolic injuries include accumulation of reactive oxygen species enhanced by oxidative stress, cochlear ischemia followed by reperfusion injury, and excitotoxicity to auditory neuron induced by excessive release of the cochlear afferent neurotransmitter, glutamate. Many studies involving therapeutic or preventive trial with antioxidants, JNK inhibitors, and NMDA antagonists have shown partial effectiveness. However, protection from noise before cochlear injury occurs is very important because damaged hair cells and auditory neurons in the mammalian cochleae are unable to regenerate.
Figures and Tables
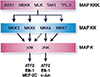 | Fig. 1Stress-activated MAPK signaling modules. The JNK and p38 MAPK are activated by dual phosphorylation on Thr and Tyr caused by members of the MAPKK group of protein kinases. The MAPKK are activated, in turn, by phosphorylation mediated by a group of MAPKKK. Stress-activated MAPK signaling modules can be created through the sequential actions of a MAPKKK, a MAPKK, and a MAPK. Ref. 30 with permission from Cell Press. |
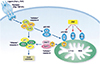 | Fig. 2Role of the JNK signaling pathway in stress-induced apoptosis. The caspase apoptotic machinery is illustrated in a simplified cartoon. Effector caspases, including caspase-3, are activated by initiator caspases that are activated by cell surface death receptors (caspase-8) and by the mitochondrial pathway (caspase-9). JNK is not required for death receptor signaling, but is required for caspase-9 activation by the mitochondrial pathway. Potential targets of JNK include members of the Bcl2 group of apoptotic regulatory proteins. Ref. 30 with permission from Cell Press. |
References
1. Phaneuf R, Hetu R. An epidemiological perspective of the causes of hearing loss among industrial workers. J Otolaryngol. 1990; 19:31–40.
2. Brink LL, Talbott EO, Burks JA, Palmer CV. Changes over time in audiometric thresholds in a group of automobile stamping and assembly workers with a hearing conservation program. AIHA J (Fairfax, Va). 2002; 63:482–487.

3. Nordmann AS, Bohne BA, Harding GW. Histopathological differences between temporary and permanent threshold shift. Hear Res. 2000; 139:13–30.

4. Liberman MC, Dodds LW. Acute ultrastructural changes in acoustic trauma: serial-section reconstruction of stereocilia and cuticular plates. Hear Res. 1987; 26:45–64.

5. Zheng XY, Henderson D, Hu BH, McFadden SL. Recovery of structure and function of inner ear afferent synapses following kainic acid excitotoxicity. Hear Res. 1997; 105:65–76.

6. Lonsbury-Martin BL, Martin GK. Noise-induced hearing loss. In : Flint PW, Haughey BH, Lund VJ, Niparko JK, Richardson MA, Robbins KT, editors. Cummings Otolaryngology Head & Neck surgery. 5th ed. Philadelphia: Mosby Elsevier;2010. p. 2140–2152.
7. Davis H. Neuroanatomy and neurophysiology in the cochlea. Trans Am Acad Ophthalmol Otolaryngol. 1952; 56:630–634.
8. Sellick PM, Patuzzi R, Johnstone BM. Measurement of basilar membrane motion in the guinea pig using the Mossbauer technique. J Acoust Soc Am. 1982; 72:131–141.

10. Turcot A, Girard SA, Courteau M, Baril J, Larocque R. Noise-induced hearing loss and combined noise and vibration exposure. Occup Med (Lond). 2015; 65:238–244.

11. Cody A, Russell I. Outer hair cells in the mammalian cochlea and noise-induced hearing loss. Nature. 1985; 315:662–665.

12. Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002; 3:248–268.

13. Bohne BA, Clark WW. Growth of hearing loss and cochlear lesion with Increasing duration of noise exposure. In : Hamernik RP, Hende.son D, Salvi RJ, editors. New Perspectives on Noise-Induced Hearing Loss. New York: Raven Press;1982. p. 283–302.
14. Hu BH, Henderson D, Nicotera TM. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res. 2002; 166:62–71.

15. Thorne PR, Nuttall AL. Laser Doppler measurements of cochlear blood flow during loud sound exposure in the guinea pig. Hear Res. 1987; 27:1–10.

16. Chen GD, Fechter LD. Potentiation of octave-band noise induced auditory impairment by carbon monoxide. Hear Res. 1999; 132:149–159.

17. Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003; 966:265–273.

18. Scheibe F, Haupt H, Ising H, Cherny L. Therapeutic effect of parenteral magnesium on noise-induced hearing loss in the guinea pig. Magnes Res. 2002; 15:27–36.
19. Attias J, Sapir S, Bresloff I, Reshef-Haran I, Ising H. Reduction in noise-induced temporary threshold shift in humans following oral magnesium intake. Clin Otolaryngol Allied Sci. 2004; 29:635–641.

21. Huang T, Cheng AG, Stupak H, Liu W, Kim A, Staecker H, et al. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci. 2000; 18:259–270.

22. Greijer A, Van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004; 57:1009–1014.

23. Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004; 1019:201–209.

24. Nicotera TM, Ding D, McFadden SL, Salvemini D, Salvi R. Paraquat-induced hair cell damage and protection with the superoxide dismutase mimetic m40403. Audiol Neurootol. 2004; 9:353–362.

25. Bielefeld EC, Hu BH, Harris KC, Henderson D. Damage and threshold shift resulting from cochlear exposure to paraquat-generated superoxide. Hear Res. 2005; 207:35–42.

26. Puel JL, Ruel J, Gervais d'Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998; 9:2109–2114.

27. Ogita K, Matsunobu T, Schacht J. Acoustic trauma enhances DNA binding of transcription factor AP-1 in the guinea pig inner ear. Neuroreport. 2000; 11:859–862.

28. Pujol R, Puel JL, Gervais d'Aldin C, Eybalin M. Pathophysiology of the glutamatergic synapses in the cochlea. Acta Otolaryngol. 1993; 113:330–334.

29. Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002; 166:33–43.

31. Nicotera TM, Hu BH, Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J Assoc Res Otolaryngol. 2003; 4:466–477.

32. Vicente-Torres M, Schacht J. A BAD link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J Neurosci Res. 2006; 83:1564–1572.

33. Kim J, Morest DK, Bohne BA. Degeneration of axons in the brainstem of the chinchilla after auditory overstimulation. Hear Res. 1997; 103:169–191.

34. Morest DK, Bohne BA. Noise-induced degeneration in the brain and representation of inner and outer hair cells. Hear Res. 1983; 9:145–151.

35. Salvi R, Henderson D, Hamernick R. Auditory fatigue: retrocochlear components. Science. 1975; 190:486–487.

36. Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear Res. 1990; 50:245–257.

37. Syka J, Rybalko N. Threshold shifts and enhancement of cortical evoked responses after noise exposure in rats. Hear Res. 2000; 139:59–68.

38. Szczepaniak WS, Moller AR. Evidence of decreased GABAergic influence on temporal integration in the inferior colliculus following acute noise exposure: a study of evoked potentials in the rat. Neurosci Lett. 1995; 196:77–80.

39. Stamper GC, Johnson TA. Auditory function in normal-hearing, noise-exposed human ears. Ear Hear. 2015; 36:172–184.

40. Brown MC, de Venecia RK, Guinan JJ Jr. Responses of medial olivocochlear neurons. Specifying the central pathways of the medial olivocochlear reflex. Exp Brain Res. 2003; 153:491–498.
41. Reiter ER, Liberman MC. Efferent-mediated protection from acoustic overexposure: relation to slow effects of olivocochlear stimulation. J Neurophysiol. 1995; 73:506–514.

42. Dallos P, He DZ, Lin X, Sziklai I, Mehta S, Evans BN. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci. 1997; 17:2212–2226.

43. Yoshida N, Kristiansen A, Liberman MC. Heat stress and protection from permanent acoustic injury in mice. J Neurosci. 1999; 19:10116–10124.

44. Niu X, Canlon B. Protective mechanisms of sound conditioning. Adv Otorhinolaryngol. 2002; 59:96–105.

46. Tahera Y, Meltser I, Johansson P, Salman H, Canlon B. Sound conditioning protects hearing by activating the hypothalamic-pituitary-adrenal axis. Neurobiol Dis. 2007; 25:189–197.

47. Kopke R, Slade MD, Jackson R, Hammill T, Fausti S, Lonsbury-Martin B, et al. Efficacy and safety of N-acetylcysteine in prevention of noise induced hearing loss: a randomized clinical trial. Hear Res. 2015; 323:40–50.

48. Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003; 966:265–273.

49. Chen Z, Ulfendahl M, Ruan R, Tan L, Duan M. Protection of auditory function against noise trauma with local caroverine administration in guinea pigs. Hear Res. 2004; 197:131–136.

50. Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, et al. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000; 20:43–50.

51. Murillo-Cuesta S, Rodríguez-de La Rosa L, Contreras J, Camarero G, Rivera T, Varela-Nieto I. Transforming growth factor β1 inhibition protects from noise-induced hearing loss. Frontiers in Aging Neuroscience. 2015; 7:32.

52. Kramer S, Dreisbach L, Lockwood J, Baldwin K, Kopke R, Scranton S, et al. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J Am Acad Audiol. 2006; 17:265–278.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download