INTRODUCTION
Stem cells are unique undifferentiated cells that have the potential for self-renewal activity while also possessing the ability to differentiate into multiple-lineages. They are categorized into embryonic stem cells (ESCs) and adult stem cells (ASCs), which include hematopoietic stem cells (HSCs) and non-hematopoietic stem cells (e.g. mesenchymal stem cells, or MSCs). ESCs that are reintroduced into blastocysts can participate in the development of all organs and tissues in the adult animal, confirming their intrinsic potency [1]. This demonstrated plasticity has made ESCs the benchmark against which stem cell potency is measured. Although ESCs have great potential, problems related to ethics, politics, and safety remain challenges to their future applications in regenerative medicine.
Recently, there has been an explosion in the number of adult stem cells that have been isolated and characterized [2]. Every tissue or organ apparently contains a stem cell population; therefore, stem cells can be easily harvested, and stem cells with low immunogenicity might be clinically used to treat disorders of more vulnerable and less accessible internal organs. For this reason, adult stem cell transplantation has already been performed to treat several degenerative diseases including liver diseases [3]. In this review, we will explain the characterization of several kinds of placenta-derived mesenchymal stem cells (PD-MSCs) and discuss animal studies of their therapeutic use in repair of injured liver with a view to their potential for clinical translation for use in regenerative medicine.
LIVER DISEASES AND THERAPEUTIC LIMITATIONS
The liver is a major vital organ that is responsible for several bodily functions, such as metabolism, detoxification, biotransformation, excretion and hormone production. Hepatic disease is one of the most common medical diagnoses. The most common causes of hepatic diseases are viral infections, toxins, alcoholism, nonalcoholic fatty liver disease, autoimmunity, and chemical abuse. Many of these conditions lead to a series of precisely regulated physiological events, despite the powerful ability of the liver to regenerate [4]. Ultimately, repetitive and continuous hepatic injuries lead to progressive liver fibrosis and ultimately cirrhosis, hepatic failure, and even hepatocellular carcinoma. As such, end-stage liver diseases such as cirrhosis represent a worldwide health problem, and currently, the only effective treatment is to transplant liver obtained from a donor into the patient. However, this treatment has many limitations, including a lack of organ donors, operative damage, the invasiveness of the procedure, the risk of immune rejection, and the high cost of transplantation. Furthermore, liver transplantation is accompanied by considerable long-term side effects, such as chronic renal failure, post-transplantation lymphoproliferative disorder and cardiovascular complications caused by immune-suppressing drugs [5]. Thus, in cases of severe hepatic dysfunction, new therapies and novel treatment strategies are warranted. Transplantation of hepatocytes as a useful therapeutic approach for liver disease has been suggested [6], however, there are several barriers preventing the use of hepatocytes in cell-based clinical applications, including difficulty in access and low cell yield [7].
STEM CELL THERAPY IN HEPATIC DISEASES
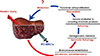
It has been reported that bone marrow is a potential source of hepatic oval cells that might be used to repair liver damage; the emerging field of stem cell therapy has shown great promise for improving the treatment of liver disease as it has the potential to promote liver repair and regeneration with fewer complications [8]. Generally, it has been reported that the natural repair system of the liver mainly depends on endogenous stem/progenitor cell pools, including hepatocytes, hepatic progenitor cells and oval cells (Fig. 1)[469]. These exogenous stem/progenitor cells are under investigation in the context of liver injury repair, and many studies have been performed in animals to analyze the efficacy and safety of this new approach with various stem cells.
Many researchers have reported on the capacity of bone marrow-mesenchymal stem cells (BM-MSCs) to differentiate into hepatocyte-like cells and produce a therapeutic effect to reduce chro-nic fibrogenesis [1011]. BM-MSCs have been reported as a potential source of hepatic oval cells, which play an important role for liver regeneration [812]. Additionally, it has been demonstrated that BM-MSCs can engraft into injured tissues such as bone marrow, lung, liver, heart, or brain and can restore tissue function by reducing inflammation and regenerating tissue [13]. Although significantly decreased fibrosis in liver tissues was observed upon treatment with BM-MSCs during a 4-week induction of CCl4 injury in rats, the mechanism by which the MSCs repaired the fibrosis remains still unclear.
There have been a considerable number of reports that BM-MSCs had no therapeutic effect in a damaged liver rat model; moreover, significant harm has been reported as they appear to transform into liver fibrogenic cells and are associated with the development of cancer [1415]. While umbilical cord-derived MSCs have shown therapeutic potential in a fibrotic rat model [16], their feasibility remains controversial due to the differences between human and animal models and the therapeutic effect of stem cell therapy in hepatic disease has been inconclusive.
CHARACTERIZATION OF PLACENTA-DERIVED MESENCHYMAL STEM CELLS
As with their adult counterparts, BM-MSCs, the fetal stem cells isolated from human umbilical cord blood are of hematopoietic derivation. Cord blood-derived stem cells have previously been clinically employed in a bone marrow transplant in 1989 [17]. They have potentials for self-renewal activity and differentiation to several hematopoietic lineages [18]. Bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells have been previously studied for cell therapy purposes [1920]. However, the potential of these adult stem cells significantly decreases in an age-dependent manner. In contrast, fetal stem cells have the advantage of being in an intermediate state between adult and embryonic stem cells [21]. Fetal stem cells have been isolated from fetal tissues, especially, multiple extra-embryonic tissues, in parallel to the gradual expansion of known stem cell sources in adults. For examples, amniotic fluid, Wharton's jelly, the amnion, the chorion, the fetal membrane and the placenta have all generated stem cells; the relative potency of each stem cell population needs to be fully determined, and this is the subject of many ongoing investigations.
Of the extra-embryonic tissues, the placenta is a large organ that plays an important role in fetal development by providing nutrition to the fetus and supporting immunological tolerance. Recently, PD-MSCs of fetal tissue origin have emerged as a new alternative source of MSCs for use in regenerative medicine. PD-MSCs demonstrate the desired capacity for self-renewal and multipotent differentiation, but they are free from ethical concerns, and are easily accessible, abundant, and strongly immunosuppressive [222324]. Furthermore, the extra-embryonic placental tissue has a large volume and is easily manipulated to yield large numbers of MSCs in comparison to MSC populations derived from bone marrow or adipose tissues [2425]. For this reason, many scientists are characterizing and investigating PD-MSCs originating from extra-embryonic tissues collected from the afterbirth, and since the afterbirth is routinely discarded, few ethical problems have emerged from the harvest of these stem cell populations. Furthermore, another advantage is that these stem cells can be isolated without the aggressive and often invasive procedures required for adult stem cell isolation. Generally, PD-MSCs can be maintained for at least 20 passages in culture and demonstrate a high proliferative capcity. Furthermore, PD-MSCs express numerous mesenchymal surface markers, including CD90 (+), CD105 (+) and HLA-DR (-), and exist in a multi-differentiated state that simultaneously expresses ectodermal, mesodermal and endodermal genes [2226]. As such, PD-MSCs have been differentiated into putative osteoblasts and adipocytes under defined in vitro conditions. Recently, the differentiation of PD-MSCs to endodermal hepatocyte-like cells was achieved in vitro [2728].
Thus, the placenta is no longer regarded as an organ to be discarded, but is instead considered as a source of valuable cells for future therapy [26], particularly in the field of regenerative medicine.
However, there are many discrepancies involved in the characterization of PD-MSCs because of their anatomical complexity, their different locations of origin or the mixed lineages of stem cells that exist in placental tissues. To clarify the definition of placenta-derived stem cells (PDSCs), Parolini and colleagues describ-ed a set of minimal criteria for defining PDSCs as follows: 1) fetal origin, 2) generation of fibroblast colony-forming units, 3) specific expression patterns of surface antigen and 4) the multipotentiality to differentiate into one or more lineages [2426].
We will discuss several types of PD-MSCs according to placental anatomy, as classified below (Fig. 2)[24]. Accordingly, cord blood- and amniotic fluid-derived stem cells and decidual layer-derived stem cells of maternal origin will not be discussed here.
1. Amniotic epithelial cells (AECs) / amnion-derived mesenchymal stem cells (AD-MSCs)
The human amniotic membrane consists of human amniotic epithelial cells (hAECs) and amniotic mesenchymal stromal cells (hAMSCs). hAECs, which are derived from the embryonic epiblast, are cuboidal-to-columnar cells that form a monolayer. The monolayer lines the fetal membrane, and hAECs secrete amniotic fluid and have direct contact with amniotic fluid. Furthermore, hAMSCs are dispersed in an extra-cellular matrix largely composed of collagen and laminin, and they are derived from the extraembryonic mesoderm. These amnion-derived stem cells display various advantageous properties, including immunomodulatory, anti-inflammatory, anti-scarring and antibacterial properties, which may explain many of the beneficial effects observed in animal models following the administration of these cells to treat a large number of inflammatory diseases [29]. In particular, transplantation of amnion-derived stem cells into the bleomycin-induced lung injury model has proven their therapeutic effects in terms of reducing pulmonary fibrosis and inflammation [30].
2. Chorionic plate-derived mesenchymal stem cells (CP-MSCs)
Mesenchymal stem cells isolated from the chorionic plate are located in the inner part of the fetal membrane of the placenta. These cells, termed CP-MSCs, have a high purity compared to other MSCs that are isolated from the placenta based on placental anatomy [924]. They express stemness-related genes (e.g. Oct-4, Nanog, and SOX2) and have the potential to differentiate into multiple lineages. Furthermore, CP-MSCs have been suggested as a material for the generation of osteogenic grafts [31], for use in neuroregeneration [32], and for treatment of hepatic disease due to their beneficial immune responses and anti-fibrotic effects [933]. However, although the above applications have been reported, there have been limited studies on the stem cell properties of CP-MSCs.
3. Umbilical cord Wharton's Jelly-derived mesenchymal stem cells (WJ-MSCs).
The umbilical cord contains two arteries, one vein, and buried Wharton's jelly, which is a proteoglycans-rich connective tissue. Recently, WJ-MSCs, also known as umbilical cord matrix-derived mesenchymal stem cells (UC-MSCs), were isolated from within the abundant extracellular matrix in which Wharton's jelly resides. The biological properties of CP-MSCs and WJ-MSCs are generally similar. However, WJ-MSCs can be obtained in larger numbers than CP-MSCs or the MSCs that can be routinely isolated from adult bone marrow and adipose tissue. Furthermore, UC-MSCs show no evidence of teratoma formation up to 12 weeks after transplantation [34], and they have immunomodulatory properties. Interestingly, WJ-MSCs show a strong capacity for chondrogenic differentiation, leading to the expression of aggrecan and cartilage oligomeric matrix protein (COMP) and the formation of a matrix-rich and multilayered mass that is accompanied by the accumulation of sulfated proteoglycans deposited by chondrocytes [24]. For these reasons, many researchers are utilizing serum-free and xeno-free culture system conditions for large-scale, clinical-grade applications of WJ-MSCs.
4. Chorionic villi-derived mesenchymal stem cells (CV-MSCs)
Generally, there are many cotyledons consisting of placental villi, in addition to the many vessels in the placenta. CV-MSCs isolated from the villi of placentas show a heterogeneous population. The heterogeneity is likely due to the presence of several different cell types, such as trophoblasts, on the surface of the villi and to the presence of stromal spindle-shaped cells, macrophages, and capillary endothelial cells on the vessels in the villi. Accordingly, the isolation of CV-MSCs may be very complex and ineffective compared with that of other placental mesenchymal stem cells. Furthermore, CV-MSCs are easily contaminated with maternal cells [35].
However, CV-MSCs express MSC and stemness markers such as Oct-4, Rex-1, GATA-4 and nestin. Additionally, they can be differentiated into mesenchymal lineages (osteocytes, adipocytes, and chondrocytes) and neuronal lineages. Although cell proliferation was greater in CV-MSCs than in adult BM-MSCs, their proliferative capacity is lower than other MSCs derived from the placenta, such as AECs, CP-MSCs, and WJ-MSCs [24].
THERAPEUTIC EFFECT OF PLACENTA-DERIVED MESENCHYMAL STEM CELLS ON LIVER DISEASES
PD-MSCs reportedly have the potential for hepatogenic differentiation and have been previously characterized [262836]. Various types of isolated PD-MSCs have been characterized with respect to their stem cell capacities and hepatogenic differentiation potential. Procedures using cytokine and growth factor cocktails confirmed the detection of appropriate hepatic markers. Although PD-MSCs have a potential for hepatogenic differentiation, there is still little data on the feasibility of using hepatogenic differentiated cells or hepatocyte-like cells derived from PD-MSCs as a therapeu-tic source because the efficiency for hepatogenic differentiation is very low and their therapeutic effects remain controversial.
Among the several PD-MSCs, several studies have shown that the application of hAECs or AD-MSCs in animal models of liver diseases such as liver fibrosis or cirrhosis can suppress collagen production via the secretion of various soluble factors [37]. Interestingly, CP-MSCs expressed stemness-related markers, three germ layer-related markers, and even albumin in their undifferentiated state [9]. These characteristics of CP-MSCs may aid in healing da-maged hepatocytes and improve injured liver function by exerting anti-fibrotic effects [93738], suggesting that these cells could be employed in cell-based therapies for liver diseases.
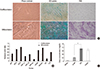
It was reported that transplantation of allogenic and xenogeneic PD-MSCs decreased neutrophil infiltration in addition to reducing fibrosis in the bleomycin-induced mouse lung fibrosis model within 2 weeks [39]. Such reports suggest the possibility of utilizing PD-MSCs as a therapeutic agent for degenerative diseases that are characterized by abnormal collagen deposition. Hepatic inflammation and fibrosis are prominent features in chronic liver diseases. During hepatic regeneration, inflammation is mediated by Kupffer cells, and fibrosis occurs primarily by activated hepatic stellate cell production of collagen [40]. Furthermore, PD-MSCs can modulate immunological responses in several degenerative diseases. MSCs have been observed to modulate immune responses in various ways as MSCs can produce a number of growth factors, cytokines and signaling molecules that can potentially suppress inflammatory responses [4142]. Interestingly, the placenta has a role in preserving fetal tolerance in immuno-suppressive environments; at so-called immune-privileged sites, multiple mechanisms cooperate to prevent immune attack [43]. Because they are derived from the placenta, hPDSCs have strong immunomodulatory properties, suggesting that hPDSC transplantation may produce weaker immune responses in recipient organs [44]. These properties are considered strongly advantageous for cell therapy using PD-MSCs in allogenic transplantation that permits stable engraftment and escape from host immune rejection. While MSCs including BM-MSCs and AD-MSCs can mobilize to injured regions [45], fetal-derived cells also have the ability to migrate across the placental and blood-brain barriers [46]. As such, one advantage of PD-MSCs is an even stronger ability to migrate. Migration, also known as "homing," involves the movement of cells to damaged tissues or organs via interactions between the MSCs and the various chemokines/cytokines secreted from the microenvironments of damaged sites. The homing ability of PD-MSCs can be stimulated further depending on culture conditions [47]. PD-MSCs were observed to home to the damaged liver after intravenous, portal vein and intrasplenic transplantation [3848]. In our previous work, the efficient homing of engrafted CP-MSCs correlated with liver regeneration in a rat model of hepatic failure according to transplantation routes (Fig. 3)[36].
CONCLUSION
Stem cell-based therapy has been spotlighted as a new strategy for treatment in several regenerative medicine therapies for degenerative diseases although they have several limitations to overcome. Herein, we discuss how PD-MSCs appear promising as an alternative stem cell source with several advantages over other stem cell sources, especially for hepatic regeneration. PD-MSCs also demonstrate unique immunomodulatory properties, suggesting that the transplantation of PD-MSCs may induce regeneration of injured tissues and produce weaker immune responses in host organs. Based on the previous review, we propose prospective therapeutic mechanisms triggered by PD-MSCs transplantation in injured liver act through multiple events as follows: 1) environmental rehabilitation through anti-fibrosis and anti-inflammation regulatory properties, 2) clearance of damaged cells and injured tissue through apoptosis and autophagy, 3) cellular protection and recycling of cellular products through autophagy, and 4) functional cell repopulation through activation of cell proliferation in injured hepatic cells. However, the appropriate guidelines and quality control for safe usage, and best therapeutic approach still remain to be developed. Although the road to using PD-MSCs as a common and safe alternative to liver transplantation is still unclear, it is very certain that PD-MSCs have many advantages and are highly attractive as an alternative source for pluripotent and multipotent cells in proposed regenerative therapies degenerative diseases including those of the liver. Therefore, further studies to understand the possibilities for using PD-MSCs in hepatic disease as an alternative to orthoptic liver transplantation are necessary and should prove to be highly informative and productive.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download