Abstract
Hepatocyte-like cells (HLCs) derived from human pluripotent stem cells are a promising cell source for drug screening and toxicity tests. Thus, various hepatic differentiating protocols have been developed, leading to a hepatic differentiation efficiency of approximately 90%. However, HLC drug metabolizing ability remains very low compared to human primary hepatocytes. In order to overcome this problem, several alternative methods, such as, co-culture, three-dimensional (3D) culture, bioreactor, nanochip-based, etc., have been developed, but optimization to produce fully functional HLCs is ongoing. Recently, our group reported that repeated exposure of HLCs to xenobiotics can improve the expression of hepatic metabolizing enzymes such as cytochrome P450s (CYPs) and glutathione S-transferases (GSTs). These data suggest that we should develop strategies for differentiating cells into mature HLCs by more closely mimicking in vivo fetal and postnatal liver development. Here, we review the current development of alternative methods for enhancing the drug metabolizing functions of HLCs derived from human embryonic stem cells, human-induced pluripotent stem cells, and mesenchymal stem cells as used for drug screening and toxicity tests.
Clinical treatment for chronic liver failure using human embryonic stem cell (hESC) and human-induced pluripotent stem cell (hiPSC)-derived hepatocyte-like cells (HLCs) is considered a pro-mising alternative method to organ transplantation. In addition to their use for treatment in liver failure, stem cell-derived HLCs have been considered for in vitro drug screening and toxicology researches [1]. Therefore, HLCs directly induced from hESCs have been intensively studied, resulting in a significant improvement in the efficiency of hepatic differentiation using human pluripotent stem cells. Albumin-positive HLCs can now be produced at the end of in vitro hepatic differentiation at levels up to 90% [2,3]. However, in spite of efforts to induce further maturation of HLCs derived from human pluripotent stem cells, the phenotype of most HLCs is more similar to fetal hepatocytes rather than fully mature hepatocytes. Critical inducing mature hepatocyte functions, such as phase I and II enzyme activity, tend to be significantly reduced in 2D-cultured HLCs (approximately <1% of human primary hepatocytes) [4,5]. Furthermore, under in vitro culture conditions, hepatobiliary transporter expression rapidly decreases [6], and most HLCs are spontaneously differentiate into various cell lineages, regardless of the differentiation protocol. Thus, at the final stage of hepatic differentiation, purification is needed to obtain highly homogenous HLCs. These key differences between HLCs and human primary hepatocytes result in limited use of HLCs as a renewable cell source of functional adult hepatocytes for cell transplantation and in vitro toxicity tests. The low metabolizing ability and limited in vitro culture duration of HLCs must be overcome prior to using HLCs for clinical applications and in vitro drug screening and toxicology researches.
A lack of knowledge regarding the developmental signaling pathways that control in vivo hepatic maturation contributes to the development of immature HLCs, which usually display a fetal phenotype. Therefore, in this review paper, we will discuss current hepatic differentiation culture systems that enhance hepatic maturation of HLCs and gain a metabolizing response against xenobiotics at a level similar to that of human primary hepatocytes.
During organogenesis, differentiation of pluripotent cells into functional hepatocytes is controlled by a complex signaling pathway of molecular events. Therefore, the most direct way of inducing hepatic differentiation is to mimic the in vivo signaling pathway present during liver development. Studies have clearly defined many of these molecular events, such as reduced Oct4 activity, which allows for the activation of FoxA2 (HNF3β), SOX17, and GATA4, a process necessary for the differentiation of endodermal cells into hepatocytes [7]. Fair et al. reported that murine ESCs in co-culture with chick cardiac mesodermal cells were successfully differentiated into early hepatocyte lineage as determined by morphology and induction of HNF3β, SOX17, and GATA4 genes [7]. Furthermore, cardiac mesodermal cells can be stimulated to form hepatic progenitor colonies in vitro. Cardiac mesoderm cells are used for hepatic differentiation because their fibroblast growth factor (FGF 1 and 2) induces competent foregut endodermal cells, which are further differentiated into hepatocytes [7]. As our knowledge of liver development during organogenesis increases, more developmentally correlated co-culture systems can be established for hepatic maturation of HLCs.
Liver parenchyma consists of 80% hepatocytes (polyploidy epithelial cells) in addition to several different types of cells such as endothelial cells, Kupffer cells, and stellate cells [8]. Together, these cells harmonize to maintain homeostatic liver functions including a response against xenobiotics, both initially as well as to repeated exposure. For example, endothelial cells often called "sinusoids" distribute blood throughout the liver. Hepatic macrophages, or Kupffer cells, ingest foreign molecules (xenobiotics and nutrients) and charge for an immune response. Additionally, stellate cells, or Ito cells, serve to store fat in the liver when they are in a resting state, where they produce rich extracellular matrix (ECM) for tissue regeneration.
The liver possesses an amazing capacity for regeneration. As a result of liver damage or hepatectomy due to liver cancer in vivo, a liver can regenerate up to approximately 75% of its original volume and double its size within four weeks. Major cytokines produced by surrounding cells of hepatocytes are tumor necrosis factor alpha (TNFα) and interleukin (IL)-1, IL-6, IL-8, IL-10 by Kupffer cells, and transforming growth factor beta (TGFβ) by activated stellate cells [9]. Many researchers speculate that this kind of regeneration is accomplished by cell-cell interactions and cytokines and growth factors produced from various surrounding cells. Based on this idea, numerous studies have examined hepatic functions of HLCs co-cultured with mesenchymal stem cells of various origins and stromal cells such as fibroblasts and endothelial cells [10]. A recent study reported that a combination of cell-cell interaction using micro-patterned dishes and co-culture with murine embryonic fibroblasts significantly increased gene expression and activity of hepatic drug metabolizing enzymes (Phase I and II) in HLCs as well as long-term culture (a month) [11].
Disadvantages of a co-culture system are the presence of difficult-to-define inducing factors as well as the need for another cell source for hepatic maturation of HLCs. Additionally, at the final stage of hepatic differentiation, a purification procedure is required to discard co-cultured cells and to harvest homogenous HLCs.
Organogenesis takes place in a 3D manner, with cell-cell interaction occurring on an ECM structure rather than a 2D flat sheet [12]. However, since Reid et al. characterized monolayer-cultured human primary hepatocytes in vitro, numerous studies have investigated ways to improve hepatic functions of not only human primary hepatocytes but also HLCs in vitro [13]. There have been several recent molecular mechanistic studies and genetic and protein profiling analysis using the 3D culture model to further understand hepatic development and enhance maturation of hepatic enzyme functionality. Generally, 3D-cultured hepatocytes have long-term expression of albumin-positive HLCs with increased hepatic metabolizing enzymes and multidrug resistance proteins (MRPs). 3D-cultured HLCs have noticeable advances in hepatic differentiation and maturation, which retain many in vivo-like properties [6].
Typical 2D culture may disrupt the complex microenvironment of an in vivo liver, resulting in the loss of liver architecture. Thus, even though the efficiency of hepatic differentiation is high in most directly induced HLCs, few studies have reported fully functional HLCs. Instead, many studies have utilized the 3D spheroid formation of hESCs, hepatoblast, or immature HLCs for hepatic maturation. Table 1 summarizes recent studies that report on either gene expression or activity of hepatic metabolizing enzymes of human pluripotent stem cell-derived HLCs using a 2D or 3D culture system. Interestingly, a few studies measure both protein levels of metabolizing enzymes and activity of induced enzymes. Various 3D culture systems have been developed for hepatic differentiation such as ultra-low attached dish, microsound bottom well plate, porous scaffold, nanopattern chip, droplet culture, and ECM.
Our group reported on HLC culturing using 3D embryonic body (EB) and 3D cultured definitive endodermal spheroid formation with lithium chloride [40,41]. However, it was difficult to prevent spontaneous differentiation during EB- or 3D culturd hepatic spheroid-mediated induction. Therefore, a purification procedure at the final stage of hepatic differentiation was needed to obtain homogenous HLCs. Most importantly, the expression of a typical hepatic cytochrome P450 (CYP)3A4 was still low, and its activity was increased -1.5 times by acetaminophen treatment. Recently, we successfully optimized our protocol and our >90% of albumin-positive HLCs were produced (Fig. 1). However, expression and activity of hepatic metabolizing enzymes in 2D cultured HLCs were much more improved but still not reached the levels of human primary hepatocytes. Therefore, we further enhanced the hepatic metabolizing ability of HLCs using a 3D culture system and repeated exposure to xenobiotics (Fig. 2,3)[8].
A disadvantage of using a 3D culture system is that HLCs, which are at the core of 3D cell clumps or spheroids, can easily undergo apoptosis due to hypoxic conditions and lack of nutrient penetration. Apoptosis of HLCs in a 3D spheroid of approximately >300 µm in diameter begins to increase. Therefore, it is important to optimize the homogenous size of 3D hepatic spheroids.
Bioreactor refers to a manufactured or engineered device producing HLCs under conditions mimicking a biologically active environment in an in vitro cell culture system. There are several different types of bioreactors such as, stirred-tank, airlift, waved, hollow-fiber etc. Key points of successful parameters for optimized bioreactors to produce massive scale HLCs are 1) detection of homogenous cell growth, 2) rate of oxygen transfer, and 3) density of maximum final cells [42]. Among the several different types of bioreactors, a simple rotating perfused bioreactor can be used with decentralized growth factors and cytokines as well as a sustained gas supply. One of the significant differences between a 3D culture system and a bioreactor is that bioreactor provides a fluidic, dynamic environment for HLCs, whereas the 3D culture system does not. The reported optimal speed of a rotating bioreactor for 3D cultured hepatic spheroids or HLCs is generally 15-20 rpm [43]. A study reported that human primary hepatocytes cultured in microgravity-simulated bioreactors formed multi-dimensional tissue-like spheroids [44]. The size of spheroids was up to 1.0 cm and the cells were arranged with biliary epithelial cells as similar as bile duct-like structures and vascular sprouts. In addition, significantly increased hepatic drug metabolizing CYPs (CYP3A5, CYP2C19, CYP2C18, CYP2C9, and CYP2D6) and MRPs were observed in HLCs cultured by a perfused 3D bioreactor, compared to 2D-cultured HLCs [12].
There are several commercially available HLCs derived from human pluripotent stem cells. Table 2 summarizes the major providers, cell types, characteristics, and servicing assays of these available HLCs [4,45,46,47,48,49]. An important factor for producing commercially available HLCs is what will be used for quality control, as they will be used for assays such as drug screening and toxicity tests. For example, proven typical hepatic metabolizing enzyme activities such as of CYP1A2 and CYP3A4 could be used as quality control standards for commercially available HLCs. The cost of a 24- or 96-well plate is approximately $2,500 if a Korean researcher orders commercially available HLCs from a US provider, and it may take three to six months to receive living HLCs on a plate. None of the major companies provide gene expression profiling of metabolizing enzymes or MRPs of their HLCs. Cellular Dynamics International (CDI), which was founded by James Thomson, reported that their iCell® hepatocytes express 1,936 absorption, distribution, metabolism, and excretion (ADME) markers in over 200 genes, which include all Food and Drug Administration (FDA)-validated genes, but the expressed genes are not reported [45]. Consequently, at this point, it is impractical to use only HLCs to predict and screen the toxicity of new drugs without using human primary hepatocytes as a control. From the point of view of pharmaceutical companies and regulatory agencies, there is no benefit to using high-priced HLCs instead of human primary hepatocytes.
Currently, European and US companies (Cellartis-European, CDI-USA, and Geron-USA) have discontinued production of HLCs. Takara Bio Europe AB acquired Cellectis-European in 2014 and Fujifilm Holdings Corp acquired CDI-USA, thus Japanese companies (ReproCell, Takara Bio Inc., and Fujifilm Holdings Corp.) fulfil the demand for HLCs in Asia, USA, and Europe. In general, bringing a new drug to market costs approximately $800 million with approximately 10% market growth each year [50]. Pharmaceutical companies invest approximately $.5 billion to develop a new drug, and it usually takes 10-15 years before their drug is brou-ght to market; nine out of ten drugs at clinical phase I will not pass marketing approval [51]. Approximately 20% of drugs demonstrate efficacy without any liver or cardiac toxicity during preclinical trials [52]. Finally, approximately 5% of candidate drugs in development can become licensed agents [53]. High research and development costs are leading to high drug prices, so it is important to develop methods for optimizing functional maturation of HLCs. At the same time, we should expand our knowledge of fundamental differences between the latest HLCs and human primary hepatocytes used for in vitro and in vivo as well as clinical application.
Many researchers are interested in improving hepatic maturation of HLCs because they may open the door to a new cell source for drug screening and toxicity tests as well as for an alternative in vitro tool for animal studies. Additionally, many studies reported on a cell-cell interaction model that may mimic liver development with molecular markers to assess timing and intensity of in vivo transcriptional events. In our most recent study, we developed a novel strategy for enhancing hepatic maturation by applying a 3D culture system with repeated exposures of HLCs to xenobiotics (Fig. 1)[8]. It was the first report to demonstrate that HLCs can be "educated," suggesting that they can acquire a "learned" response to hepatotoxins.
Our strategy is based on the idea that hepatic metabolizing ability (activity of Phase I and II) has been primarily accomplished within the first three years of life. A neonate's liver has only -20% of the hepatocytes that will be present in the adult hepatocytes [54]. Therefore, the metabolizing rate and function of neonatal hepatocytes against to xenobiotics are low. Increased sensitivity of hepatocytes postnatally to xenobiotics may be due to repeated exposure to various xenobiotics, including those acquired through the diet early in life. This epigenetic modification method could induce expression and activities of hepatic metabolizing enzymes and thus produce highly functional HLCs for clinical application and in vitro toxicity screening.
According to our data, in addition to the ethnic variations between hESCs and hiPSCs, there are also biological variations regarding the efficiency of hepatic differentiation and gene expression profiling of hepatic metabolizing enzymes. Furthermore, the efficacy of one of the hiPSCs tested in our previous study was approximately 40%, indicating that potential tissue-specific genotypic variations of different hiPSC-derived HLCs exist. Therefore, when analyzing hepatotoxicity using HLCs, the ethnic and biological background of HLCs should be taken into account when making a rational and comprehensive decision.
Recently, in addition to HLCs derived from hESCs and hiPSCs, a population of liver stem cells has been identified by Nusse's group [14]. Liver stem cells can proliferate and differentiate, giving rise to fully functional hepatocytes used to regenerate liver tissue. If we analyze and compare the similarities and differences among HLCs derived from hESCs and hiPSCs as well as newly identified liver stem cells, it may provide us with useful clues regarding maturating HLCs. In addition, it may provide information on how to delay or prevent degeneration of hepatic function and loss of hepatic characteristics in human primary hepatocytes.
The main concern in hepatic differentiation using human pluripotent stem cells is how to enhance the drug metabolizing ability of HLCs. Of the major drug metabolizing abilities of HLCs, phase I and II enzyme activity and MRPs are the focus of improvement efforts. Several previous studies have developed assays for these hepatic enzymes and MRPs in human pluripotent stem cell-derived HLCs such as co-culture, 3D culture, and bioreactor. Mimicking in vivo liver development and functional maturation is the primary goal of these efforts. Unfortunately, many studies did not report promising outcomes, and their results indicate that there is still a long way to go towards enhancing drug metabolizing enzymes and MRPs in HLCs so that they are comparable to human primary hepatocytes. A key future task is developing a protocol to differentiate functionally mature hepatocytes derived from human pluripotent stem cells in sufficient numbers for clinical application. In order to accomplish this, a more solid understanding of cellular signal transduction, cell-cell interactions, cell-matrix interactions, and hepatic enzyme expression during liver development needs to be acquired, accompanied by stem cell research. In addition, functional comparisons between HLCs and human primary hepatocytes, liver stem cells, and in vivo liver are needed. Standardization of functional quality control of various phenotypes of HLCs for drug screening and testing (efficacy and toxicity) is needed for therapeutic application in the near future.
Figures and Tables
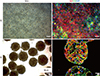 | Fig. 1(A) Characterization of 2D and 3D cultured HLCs. Phase contrast images of HLCs and (B) immunofluorescent images of hepatic markers (ALB: red and CK18: green) in HLCs at the final stage III of hepatic differentiation. Note that 3D hepatic spheroids were formed and further differentiated from single-cell dissociated 2D hepablast. HLCs, hepatocyte-like cells; 3D, three-dimensional. |
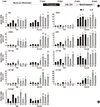 | Fig. 2Enhanced hepatic metabolizing HLCs using 3D culture systems and repeated exposure to xenobiotics. (A) Schematic representation of repeated exposure of 2D-cultured HLCs and 3D hepatic spheroids to xenobiotics. Note that the HLCs and hepatic spheroids derived from BGO1 hESCs were exposed to each xenobiotic concentration at the end of stage III. The xenobiotic treatment was then withdrawn for 2 days followed by a second exposure for 2 days. (B) qPCR analysis of ALB and three transcription factors known for their expression in mature hepatocytes (PROX1, C/EBPa, and ATF5) and phase I enzymes (CYP1A2, CYP2D6, CYP2C9, CYP3A4, and CYP3A7) in 2D-cultured HLCs after repeated exposure to xenobiotics. The 'a' denotes statistical significance. Control (C, untreated HLCs); the 'b' denotes statistical significance, compared to the first exposure of its own group. Phenobarbital (PB, widely prescribed as an anti-seizure medication in children); Acetaminophen (AP, a common cold medication); rifampicin (RIF, an antibiotic used to treat infections). OSM, oncostatin M and DEX, dexamethasone. Ref. 2 with permission from Oxford University Press. |
 | Fig. 3Comparison of hepatic gene expression among 2D-cultured HLCs (2D), 3D-cultured hepatic spheroids at day 6 of stage III (3D), and three different human primary hepatocytes 24 hours after in vitro culture (2D) using qPCR analysis. Each of the 2D-cultured human primary hepatocytes (green bars) were obtained from Caucasian (C), African American (AA), and Asian (A) subjects. Statistical significance is reported as 'a' for statistical significance of gene expression between 2D HLCs and 3D hepatic spheroids, 'b' for statistical significance of gene expression between 3D hepatic spheroids and 2D human primary hepatocytes, and 'c' for statistical significance of gene expression between 2D HLCs and 2D human primary hepatocytes. (A) Expression of ALB (albumin), PROX1, and AFP. (B) Expression of nuclear receptor (NR), which regulate the expression of key hepatic CYP genes. (C) Gene expression of various isoform GSTs, which are part of the GST phase II metabolic enzyme family. (D) Gene expression of hepatic CYPs. Data are presented as the means 6SD of 4 separate experiments. Ref. 2 with permission from Oxford University Press. |
ACKNOWLEDGMENTS
This work was supported by Basic Research Program through the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (the Ministry of Science, ICT & Future Planning, MSIP, No. 2015R1C1A1A02036905) and a Research Fellowship Grant from Korea University for Jiyou Han, MSIP (No. 2012M3A9C7050139, No. 2012M3A9B4028636), and the School of Life Science and Biotechnology for BK21PLUS for Jong-Hoon Kim.
References
1. Kia R, Sison RL, Heslop J, Kitteringham NR, Hanley N, Mills JS, et al. Stem cell-derived hepatocytes as a predictive model for drug-induced liver injury: are we there yet? Br J Clin Pharmacol. 2013; 75:885–896.

2. Kim JH, Jang YJ, An SY, Son J, Lee J, Lee G, et al. Enhanced metabolizing activity of human ES cell-derived hepatocytes using a 3D culture system with repeated exposures to xenobiotics. Toxicol Sci. 2015; 147:190–206.

3. Subramanian K, Owens DJ, Raju R, Firpo M, O'Brien TD, Verfaillie CM, et al. Spheroid culture for enhanced differentiation of human embryonic stem cells to hepatocyte-like cells. Stem Cells Dev. 2014; 23:124–131.

4. The world's first commercial human iPSC-derived hepatocytes [Internet]. Yokohama (JPN): ReproCELL;c2012. cited 2015 Oct 20. Available from: https://www.reprocell.com/en/products/cell-biorepository/reprohepato/.
5. Schwartz RE, Fleming HE, Khetani SR, Bhatia SN. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol Adv. 2014; 32:504–513.

6. Meng Q, Haque A, Hexig B, Akaike T. The differentiation and isolation of mouse embryonic stem cells toward hepatocytes using galactose-carrying substrata. Biomaterials. 2012; 33:1414–1427.

7. Fair JH, Cairns BA, Lapaglia M, Wang J, Meyer AA, Kim H, et al. Induction of hepatic differentiation in embryonic stem cells by co-culture with embryonic cardiac mesoderm. Surgery. 2003; 134:189–196.

8. LeCluyse EL, Witek RP, Andersen ME, Powers MJ. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol. 2012; 42:501–548.

9. Neuman MG, Brenner DA, Rehermann B, Taieb J, Chollet-Martin S, Cohard M, et al. Mechanisms of alcoholic liver disease: cytokines. Alcohol Clin Exp Res. 2001; 25:251S–253S.

10. Ishii T, Yasuchika K, Fukumitsu K, Kawamoto T, Kawamura-Saitoh M, Amagai Y, et al. In vitro hepatic maturation of human embryonic stem cells by using a mesenchymal cell line derived from murine fetal livers. Cell Tissue Res. 2010; 339:505–512.

11. Berger DR, Ware BR, Davidson MD, Allsup SR, Khetani SR. Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology. 2015; 61:1370–1381.

12. Setty Y, Cohen IR, Dor Y, Harel D. Four-dimensional realistic modeling of pancreatic organogenesis. Proc Natl Acad Sci U S A. 2008; 105:20374–20379.

13. Reid LM, Gaitmaitan Z, Arias I, Ponce P, Rojkind M. Long-term cultures of normal rat hepoatocytes on liver biomatrix. Ann N Y Acad Sci. 1980; 349:70–76.
14. Park HJ, Choi YJ, Kim JW, Chun HS, Im I, Yoon S, et al. Differences in the epigenetic regulation of cytochrome P450 genes between human embryonic stem cell-derived hepatocytes and primary hepatocytes. PLoS One. 2015; 10:e0132992.

15. Siller R, Greenhough S, Naumovska E, Sullivan GJ. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem Cell Reports. 2015; 4:939–952.

16. Buyl K, De Kock J, Najar M, Lagneaux L, Branson S, Rogiers V, et al. Characterization of hepatic markers in human Wharton's Jelly-derived mesenchymal stem cells. Toxicol In Vitro. 2014; 28:113–119.

17. Farzaneh Z, Pakzad M, Vosough M, Pournasr B, Baharvand H. Differentiation of human embryonic stem cells to hepatocyte-like cells on a new developed xeno-free extracellular matrix. Histochem Cell Biol. 2014; 142:217–226.

18. Vaghjiani V, Vaithilingam V, Saraswati I, Sali A, Murthi P, Kalionis B, et al. Hepatocyte-like cells derived from human amniotic epithelial cells can be encapsulated without loss of viability or function in vitro. Stem Cells Dev. 2014; 23:866–876.

19. Subramanian K, Owens DJ, Raju R, Firpo M, O'Brien TD, Verfaillie CM, et al. Spheroid culture for enhanced differentiation of human embryonic stem cells to hepatocyte-like cells. Stem Cells Dev. 2014; 23:124–131.

20. Park Y, Chen Y, Ordovas L, Verfaillie CM. Hepatic differentiation of human embryonic stem cells on microcarriers. J Biotechnol. 2014; 174:39–48.

21. Li X, Yuan J, Li W, Liu S, Hua M, Lu X, et al. Direct differentiation of homogeneous human adipose stem cells into functional hepatocytes by mimicking liver embryogenesis. J Cell Physiol. 2014; 229:801–812.

22. Gieseck RL, Hannan NR, Bort R, Hanley NA, Drake RA, Cameron GW, et al. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS One. 2014; 9:e86372.

23. Kondo Y, Iwao T, Nakamura K, Sasaki T, Takahashi S, Kamada N, et al. An efficient method for differentiation of human induced pluripotent stem cells into hepatocyte-like cells retaining drug metabolizing activity. Drug Metab Pharmacokinet. 2014; 29:237–243.

24. Ulvestad M, Nordell P, Asplund A, Rehnström M, Jacobsson S, Holmgren G, et al. Drug metabolizing enzyme and transporter protein profiles of hepatocytes derived from human embryonic and induced pluripotent stem cells. Biochem Pharmacol. 2013; 86:691–702.

25. Yanagida A, Ito K, Chikada H, Nakauchi H, Kamiya A. An in vitro expansion system for generation of human iPS cell-derived hepatic progenitor-like cells exhibiting a bipotent differentiation potential. PLoS One. 2013; 8:e67541.

26. Ma X, Duan Y, Tschudy-Seney B, Roll G, Behbahan IS, Ahuja TP, et al. Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem Cells Transl Med. 2013; 2:409–419.

27. Cheong HH, Masilamani J, Chan CY, Chan SY, Phan TT. Metabolically functional hepatocyte-like cells from human umbilical cord lining epithelial cells. Assay Drug Dev Technol. 2013; 11:130–138.

28. Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, et al. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B. 2013; 14:961–972.

29. Dong X, Pan R, Zhang H, Yang C, Shao J, Xiang L. Modification of histone acetylation facilitates hepatic differentiation of human bone marrow mesenchymal stem cells. PLoS One. 2013; 8:e63405.

30. Sgodda M, Mobus S, Hoepfner J, Sharma AD, Schambach A, Greber B, et al. Improved hepatic differentiation strategies for human induced pluripotent stem cells. Curr Mol Med. 2013; 13:842–855.

31. Yang G, Si-Tayeb K, Corbineau S, Vernet R, Gayon R, Dianat N, et al. Integration-deficient lentivectors: an effective strategy to purify and differentiate human embryonic stem cell-derived hepatic progenitors. BMC Biol. 2013; 11:86.

32. Sasaki T, Takahashi S, Numata Y, Narita M, Tanaka Y, Kumagai T, et al. Hepatocyte nuclear factor 6 activates the transcription of CYP3A4 in hepatocyte-like cells differentiated from human induced pluripotent stem cells. Drug Metab Pharmacokinet. 2013; 28:250–259.

33. Seeliger C, Culmes M, Schyschka L, Yan X, Damm G, Wang Z, et al. Decrease of global methylation improves significantly hepatic differentiation of Ad-MSCs: possible future application for urea detoxification. Cell Transplant. 2013; 22:119–131.

34. Kostadinova R, Boess F, Applegate D, Suter L, Weiser T, Singer T, et al. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol Appl Pharmacol. 2013; 268:1–16.

35. Ramasamy TS, Yu JS, Selden C, Hodgson H, Cui W. Application of three-dimensional culture conditions to human embryonic stem cell-derived definitive endoderm cells enhances hepatocyte differentiation and functionality. Tissue Eng Part A. 2013; 19:360–367.

36. Vosough M, Omidinia E, Kadivar M, Shokrgozar MA, Pournasr B, Aghdami N, et al. Generation of functional hepatocyte-like cells from human pluripotent stem cells in a scalable suspension culture. Stem Cells Dev. 2013; 22:2693–2705.

37. Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012; 55:1193–1203.

38. Yu Y, Liu H, Ikeda Y, Amiot BP, Rinaldo P, Duncan SA, et al. Hepatocyte-like cells differentiated from human induced pluripotent stem cells: relevance to cellular therapies. Stem Cell Res. 2012; 9:196–207.

39. Hua M, Zhang W, Li W, Li X, Liu B, Lu X, et al. Molecular mechanisms regulating the establishment of hepatocyte polarity during human hepatic progenitor cell differentiation into a functional hepatocyte-like phenotype. J Cell Sci. 2012; 125:5800–5810.

40. Kim SE, An SY, Woo DH, Han J, Kim JH, Jang YJ, et al. Engraftment potential of spheroid-forming hepatic endoderm derived from human embryonic stem cells. Stem cells Dev. 2013; 22:1818–1829.

41. Woo DH, Kim SK, Lim HJ, Heo J, Park HS, Kang GY, et al. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology. 2012; 142:602–611.

42. Wang D, Liu W, Han B, Xu R. The bioreactor: a powerful tool for large-scale culture of animal cells. Curr Pharm Biotechnol. 2005; 6:397–403.

43. Fridley KM, Fernandez I, Li MT, Kettlewell RB, Roy K. Unique differentiation profile of mouse embryonic stem cells in rotary and stirred tank bioreactors. Tissue Eng Part A. 2010; 4:3285–3298.

44. Yoffe B, Darlington GJ, Soriano HE, Krishnan B, Risin D, Pellis NR, et al. Cultures of human liver cells in simulated microgravity environment. Adv Space Res. 1999; 24:829–836.

45. iCell® Hepatocytes [Internet]. USA: Cellular dynamics international;c2013. cited 2015 Oct 20. Available from: http://cellulardynamics.com/products-services/icell-products/icell-hepatocytes/.
46. Stem cell techonology: liversafe 3DTM [Internet]. San Francisco (CA): VistaGen Therapeutics;cited 2015 Oct 20. Available from: http://www.vistagen.com/?page_id=113/.
47. Stem assays: hepatoGLOTM [Internet]. Colorado Springs (CO): Hemo Genix Changing the Pradigm;cited 2015 Oct 20. Available from: http://www.hemogenix.com/prod_STEM1.php?tab=6/.
48. Geron. [Internet]. cited 2015 Oct 20. Available from: http://www.geron.com/.
49. Stem cell research: human stem cell derived hepatocytes [Internet]. Otsu (JPN): TaKaRa, Clontech;cited 2015 Oct 20. Available from: http://www.cellartis.com/stem-cell-research/.
50. DiMasi JA. The value of improving the productivity of the drug development process: faster times and better decisions. Pharmacoeconomics. 2002; 20:Suppl 3. 1–10.

51. Chapman KL, Holzgrefe H, Black LE, Brown M, Chellman G, Copeman C, et al. Pharmaceutical toxicology: designing studies to reduce animal use, while maximizing human translation. Regul Toxicol Pharmacol. 2013; 66:88–103.

52. Hutchinson L, Kirk R. High drug attrition rates--where are we going wrong? Nat Rev Clin Oncol. 2011; 8:189–190.

53. Potta SP, Šarić T, Heke M, Bahudhanapati H, Hescheler J. Human Pluripotent stem cell applications in drug discovery and toxicology - an overview. In : Atwood CS, Meethal SV, editors. Pluripotent stem cell biology - advances in mechanisms, methods and models. Atlanta: Intech;2014. p. 181–196.
54. Wanless IR. Anatomy, histology, embryology, and developmental anomalies of the liver. In : Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger & Fordtran's Gastrointestinal and Liver Disease. 7th ed. Philadelphia: Saunders;2002. p. 1195–1201.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download