Abstract
There have been conflicting reports on the continuation of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) in patients with newly developed or progressive brain metastasis of non-small cell lung cancer (NSCLC). Patients with newly developed or progressive intracranial lesions, but who maintained well-controlled extracranial disease during erlotinib treatment, were enrolled in this study. The proposed therapy included stereotactic radiosurgery (SRS), whole brain radiotherapy (WBRT), and/or surgical resection for intracranial lesions. Erlotinib treatment was continued simultaneously unless extracranial disease progressed. The evaluation of both extra- and intra-cranial lesions was performed every 3 months. From October 2009 to June 2012, 14 patients were enrolled in this pilot study. For intracranial disease, 4 patients received SRS alone, 7 patients received both SRS and WBRT, 2 patients received SRS, WBRT and surgical resection, and 1 patient received no local therapy due to the presence of asymptomatic lesions. Of the patients with extracranial disease who were placed on continued erlotinib therapy, 6 patients (42.9%) showed partial response (PR), while 7 patients (50.0%) remained in stable disease (SD). The progression-free survival (PFS) of extracranial and intracranial disease was 11.1 (range 1.6-34.6) and 10.2 (range 1.5-34.6) months, respectively. In 5 cases, brain lesions relapsed before the progression of extracranial disease. Overall survival (OS) was 22.6 (range 2.1-50.4) months. For NSCLC patients with progression of only intracranial disease during erlotinib treatment, the continuation of erlotinib in combination with local therapy to brain metastases can be an effective treatment option.
Over the past several decades, chemotherapy has been the main treatment option for advanced non-small cell lung cancer (NSCLC). In metastatic disease, chemotherapy improves treatment outcomes with a median overall survival time of 9 to 12 months [12]. However, despite continuous advances in the treatment of NSCLC, the involvement of brain metastases remains a grave complication that results in shorter survival and marked deterioration in quality of life [3]. For therapeutic approach to patients with brain metastases, whole brain radiotherapy (WBRT) has been a cornerstone treatment for decades [45]. Recently, local therapies such as surgical resection and stereotactic radiosurgery (SRS) have been improving prognosis and survival of these patients [678]. On the other hand, systemic therapy also remains an essential part of treatment for disseminated disease [59].
The epidermal growth factor receptor (EGFR) is a transmembrane receptor expressed at high levels in a variety of solid tumors, including NSCLC. EGFR has been implicated in the control of cell survival, proliferation, metastasis, and angiogenesis [10]. Erlotinib is an oral epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) that has demonstrated efficacy in patients with advanced NSCLC harboring activating EGFR mutations [1112]. In addition, erlotinib prolongs survival in NSCLC patients with wild type EGFR even after the failure of at least one prior chemotherapy regimen [1314]. However, the penetration rate of erlotinib into the cerebrospinal fluid (CSF) is relatively low, 2.77% to 4.4% of plasma level [1516]. Indeed, several retrospective studies have revealed a high incidence of disease recurrence in the brain and leptomeninges after an initial response to EGFR-TKIs [17]. Interestingly, however, the majority of patients who had disease recurrence in the central nervous system (CNS) had achieved good control of other disease sites [18]. This discrepant response between the brain and extracranial sites has been a challenging clinical problem because of the lack of effective therapies. Systemic chemotherapy is largely unsuccessful for two main reasons: drugs cannot effectively penetrate the blood-brain barrier, and NSCLC is only poorly to moderately sensitive to systemic treatment [9]. In spite of this, even in the presence of multiple metastases, some patients can be effectively treated by surgical resection, SRS or WBRT, although the median survival after WBRT is only 3-4 months [678]. Recently, the use of erlotinib in combination with WBRT and other local therapies has demonstrated favorable outcomes in patients [19].
Thus, we conducted this pilot study to determine whether the continuation of erlotinib treatment can maintain controlled extracranial disease, as long as cranial lesions are controlled separately by conventional treatment modalities such as surgical resection, SRS, and WBRT.
The patients enrolled in this study had newly developed or progressive brain lesions while in good control of extracranial NSCLC lesions during erlotinib treatment. Eligible patients were 18 years or older, with histologically or cytologically proven NSCLC, and were receiving erlotinib as a salvage therapy (i.e. second line). Entry criteria included: Eastern Cooperative Oncology Group (ECOG) performance status ≤2; at least one unidimensionally measurable or evaluable systemic lesion (brain lesion with a diameter >10 mm using brain magnetic resonance imaging (MRI); adequate hematologic function (Hgb ≥9 g/dL, platelet ≥100,000/µL, absolute neutrophil count ≥1,500/µL); adequate hepatic function (serum bilirubin ≤1.5×upper normal limit (UNL), AST (SGOT) and ALT (SGPT) ≤2.5×UNL), alkaline phosphatase ≤5×UNL (except in case of bone metastasis without any liver disease); and adequate renal function (serum creatinine ≤1×UNL or calculated creatinine clearance ≥60 mL/min).
Patients who had leptomeningeal metastases, acute severe infection requiring antibiotic therapy, significant cardiovascular disease, uncontrolled diabetes mellitus, or severe ophthalmologic disease were ineligible for this study.
All patients gave written informed consent prior to study-specific screening procedures, with the understanding that the patient had the right to withdraw from the study at any time, without prejudice. This study was approved by the Institutional Review Board.
Erlotinib was administered at a dose of 150 mg/day, from day 1 to day 28. However, if the patient was already receiving a dose of 100 mg/day before the enrollment, we continued treatment using this dose. Each cycle was repeated every 4 weeks. Erlotinib was administered continuously unless the disease progressed, or drug toxicity reached unacceptable levels, or by a patient's refusal. A baseline scan was performed at the beginning of treatment, followed by subsequent scans every 12 weeks (3 cycles) of treatment. Chest computed tomography (CT) and brain MRI scans were performed in order to identify extracranial and intracranial lesions, respectively. As noted, treatment was continuous unless progressive disease (PD) was evident. For extracranial lesions, an additional CT scan was performed after the first cycle. Only when disease progression was suspected were follow-up assessments done before the scheduled time point. If stable disease was maintained for greater than 1 year, then follow-up office visits were scheduled for once every 3 months.
The primary objective of this treatment regimen was to attain progression-free survival (PFS) of extracranial disease with good control of intracranial lesions. This would be achieved separately by using conventional treatment modalities including surgical resection, SRS, or WBRT. The secondary objectives were PFS of intracranial disease and enhanced overall survival (OS). All the treatment procedures for intracranial disease were authorized by the principal investigator, upon consultation with therapeutic radiologists and neurosurgeons. Tumor response, progression, and survival information recorded in the clinical database was based on the assessment of the principal investigator managing the patient. Response and progression measurements were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 guidelines. Estimates of objective tumor response rates and response category frequencies were computed within 95% confidence intervals.
Toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 guidelines. If deemed too toxic, treatment was delayed for 1 week, and then followed by a 50 mg daily dose reduction of erlotinib (depending on the schedule). If toxicity resulted in a dosing delay or interruption of study drug for more than 3 weeks, then the patient was withdrawn from the study for toxicity reasons.
Response rates and clinical characteristics were evaluated using the chi-square test or the Fisher's exact test for categorical variables. The PFS and OS were calculated using the Kaplan-Meier method. The progression-free survival of extracranial disease (PFSed) and intracranial disease (PFSid) were calculated as the period from the first day of erlotinib reinitiation after study enrollment to the date of extracranial and intracranial disease progression, respectively, or the date of last follow-up. The OS was calculated as the period from the first day of erlotinib reinitiation after study enrollment to the date of death, or the date of last follow-up.
From October 2009 to June 2012, 14 patients were enrolled in the Samsung Medical Center, Seoul. Patient demographics and baseline characteristics are listed in Table 1. The median age of all patients was 61 years. There were 9 male and 5 female patients. The histology of 13 patients was adenocarcinoma while 1 patient had adeno-squamous carcinoma. All patients had received platinum-based doublet chemotherapy before erlotinib treatment. Of the 14 patients, 12 had pre-existing brain lesions, while 2 were newly diagnosed with brain metastases. Eleven of 12 patients with pre-existing brain metastases had received local treatments before study enrollment, SRS in 11, WBRT in 5, and surgical resection in 3 patients. Sequencing analysis of the EGFR gene from the DNA of primary lung tumor tissue was performed in 11 of 14 patients. Six of them had activating EGFR mutations; 4 had an exon 19 deletion, and 2 had an L858R mutation. One of the patients with an L858R mutation also had a T790M mutation. At the time of study enrollment, the median duration of erlotinib treatment for all patients was 12.3 (range 2.2-38.2) months, and 13 patients showed partial responses to erlotinib. For intracranial disease, 4 patients received SRS alone, 7 patients received both SRS and WBRT, 2 patients received both SRS and WBRT with surgical resection, and 1 patient received no therapy to the brain due to asymptomatic lesions.
The disease control rate (DCR) for combined extracranial and intracranial disease was 92.9%: 6 patients (42.9%) showed partial response (PR) and 7 patients (50%) remained stable disease (SD). For extracranial disease, 12 patients (85.7%) remained in SD and one patient showed PR. The progression-free survival of extracranial disease (PFSed) and intracranial disease (PFSid) was 11.1 (range 1.6-34.6) and 10.2 (range 1.5-34.6) months, respectively. The PFSed in each patient is shown in Fig. 1. In five cases, intracranial disease progressed earlier than extracranial disease. After confirming the progression of intracranial disease, SRS was performed as a salvage therapy in all but one patient. The median overall survival time was 22.6 (range 2.1-50.4) months (Fig. 2). There was no significant difference in PFS according to the presence of activating EGFR mutations.
In this pilot study, we found that continuation of erlotinib can maintain controlled extracranial disease without change to chemotherapy as long as brain metastasis is controlled by local therapy in NSCLC patients who developed brain only progression. The median extracranial PFS was 11.1 months (range 1.6-34.6) with continuation of erlotinib. Some of the patients can continue erlotinib receiving multiple local therapies for brain metastasis while extracranial lesion remains stable (Fig. 1).
It has been reported that brain metastasis is a frequent site of disease recurrence or progression in patients after an initial response to EGFR-TKI, regardless of disease control in extracranial disease [17]. It can be hypothesized that the discrepant response between the brain and extracranial sites is due to several reasons; low penetration rate of EGFR-TKIs to CSF, longer survival and oligoclonality of the tumor; whereby some subclones are sensitive to EGFR-TKIs, while others harbor modified genes that confer resistance to EGFR-TKIs.
The routine clinical practice for NSCLC patients treated with EGFR-TKI who developed brain progression is discontinuation of EGFR-TKI and change to systemic chemotherapy. However, several studies have reported on the rapid progression of systemic disease after the discontinuation of EGFR-TKIs [20]. In general, the prognosis of patients with brain progression who initially responded to EGFR-TKI is poor. Omuro et al reported that the median overall survival for those patients with or without CNS metastases was 15 months and 23 months, respectively [17]. The majority of studies performed in the past were concerned primarily with the initial management of brain metastases in NSCLC, while studies to determine the treatment strategy for recurrent/progressive CNS disease with targeted therapy were lacking [19202122].
There is gradually accumulating evidence showing the clinical benefits of continuing EGFR-TKIs beyond disease progression. Riely et al. reported on a prospective trial of 10 patients who had previously responded to erlotinib or gefitinib, and who benefited from continued EGFR-TKI treatment despite disease progression as evaluated by RECIST [23]. Several strategies were applied in this situation: switching from gefitinib to erlotinib, retrial of the same EGFR-TKI after a drug 'holiday', and high-dose therapy of gefitinib or erlotinib [24252627]. It seems that gefitinib failed to show any benefit after disease progression compared to erlotinib treatment because the recommended daily dose of erlotinib is much nearer to the maximum tolerated dose (MTD) than that of gefitinib [28]. Shukuya et al reported that retrospective analysis of continuous EGFR-TKI administration following radiotherapy for NSCLC patients with isolated CNS failure showed 41% of response rate and 76% of disease control rate of CNS lesions with 403 days of overall survival, which correlates well with the results of the present study [29]. Currently, study of first-line erlotinib until and beyond RECIST progression in Asian patients with EGFR mutation-positive NSCLC (ASPIRATION; NCT01310036) is ongoing. In this study, the PFS1 (time to RECIST progression or death) and PFS2 (time to off-erlotinib progression if erlotinib was extended beyond RECIST progression) will be assessed. The results of continuation of erlotinib especially in CNS progression are awaiting.
The present prospective study showed a higher disease control rate (DCR) and prolonged survival time with continued erlotinib treatment plus local control of brain metastases. Notably, the median overall survival time was 22.6 months, which is much higher than that reported in previous studies using EGFR-TKIs in NSCLC patients with CNS disease, where the median overall survival was 6 to 12 months [1930].
This study has several limitations. This study was designed as a single-arm study because an appropriate control group was hard to establish, as there is no proven effective alternative treatment strategy for this setting. Only 14 patients were investigated, making it difficult to obtain statistically significant results from the subgroup analysis. Nevertheless, this is the first prospective observational study of NSCLC patients with progressive brain metastases who are undergoing targeted therapy.
In conclusion, for patients with progression of only intracranial disease during erlotinib treatment, the continuation of erlotinib in combination with local therapy to brain metastases can be an effective treatment option.
Figures and Tables
 | Fig. 1Progression-free survival (PFS) of extracranial disease. PFS of extracranial disease calculated as the period from the first day of erlotinib reinitiation after study enrollment to the date of extracranial disease progression or the date of last follow-up. *EGFR mutation positivity. |
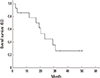 | Fig. 2Kaplan-Meier curve of overall survival (OS). OS calculated as the period from the first day of erlotinib reinitiation after study enrollment to the date of death or the date of last follow-up. |
References
1. Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004; 22:3852–3859.

2. Hotta K, Fujiwara Y, Matsuo K, Suzuki T, Kiura K, Tabata M, et al. Recent improvement in the survival of patients with advanced nonsmall cell lung cancer enrolled in phase III trials of first-line, systemic chemotherapy. Cancer. 2007; 109:939–948.

3. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012; 30:419–425.

4. Gaspar LE, Mehta MP, Patchell RA, Burri SH, Robinson PD, Morris RE, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010; 96:17–32.

5. Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23:Suppl 7. vii56–vii64.

6. Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol. 2009; 92:275–282.

7. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004; 363:1665–1672.

8. Scoccianti S, Ricardi U. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol. 2012; 102:168–179.

9. Mehta MP, Paleologos NA, Mikkelsen T, Robinson PD, Ammirati M, Andrews DW, et al. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010; 96:71–83.

10. Cedres S, Prat A, Martinez P, Pallisa E, Sala G, Andreu J, et al. Clinical surrogate markers of survival in advanced non-small cell lung cancer (NSCLC) patients treated with second-third line erlotinib. Lung Cancer. 2009; 66:257–261.

11. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–742.

12. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–246.
13. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005; 353:123–132.

14. Reck M, van Zandwijk N, Gridelli C, Baliko Z, Rischin D, Allan S, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010; 5:1616–1622.

15. Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012; 70:399–405.

16. Deng Y, Feng W, Wu J, Chen Z, Tang Y, Zhang H, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol. 2014; 2:116–120.

17. Omuro AM, Kris MG, Miller VA, Franceschi E, Shah N, Milton DT, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005; 103:2344–2348.

18. Lee YJ, Choi HJ, Kim SK, Chang J, Moon JW, Park IK, et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer. 2010; 116:1336–1343.

19. Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013; 31:895–902.

20. Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011; 17:6298–6303.

22. Lee JC, Jang SH, Lee KY, Kim YC. Treatment of Non-small Cell Lung Carcinoma after Failure of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. Cancer Res Treat. 2013; 45:79–85.

23. Riely GJ, Kris MG, Zhao B, Akhurst T, Milton DT, Moore E, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007; 13:5150–5155.

24. Cho BC, Im CK, Park MS, Kim SK, Chang J, Park JP, et al. Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J Clin Oncol. 2007; 25:2528–2533.

25. Becker A, Crombag L, Heideman DA, Thunnissen FB, van Wijk AW, Postmus PE, et al. Retreatment with erlotinib: Regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer. 2011; 47:2603–2606.

26. Jackman DM, Holmes AJ, Lindeman N, Wen PY, Kesari S, Borras AM, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006; 24:4517–4520.

27. Kuiper JL, Smit EF. High-dose, pulsatile erlotinib in two NSCLC patients with leptomeningeal metastases--one with a remarkable thoracic response as well. Lung Cancer. 2013; 80:102–105.

28. Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001; 19:3267–3279.

29. Shukuya T, Takahashi T, Naito T, Kaira R, Ono A, Nakamura Y, et al. Continuous EGFR-TKI administration following radiotherapy for non-small cell lung cancer patients with isolated CNS failure. Lung Cancer. 2011; 74:457–461.

30. McHaffie DR, Chabot P, Dagnault A, Suh JH, Fortin MA, Chang E, et al. Safety and feasibility of motexafin gadolinium administration with whole brain radiation therapy and stereotactic radiosurgery boost in the treatment of </= 6 brain metastases: a multi-institutional phase II trial. J Neurooncol. 2011; 105:301–308.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download